Adsorption and gas-sensitive properties of TM (Rh, Pd, Pt) modified Ti3C2F2 for SO2, NO2 and NH3 gas molecules: A DFT study
IF 1.8
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In the present investigation, the adsorption and gas-sensitive properties of industrial toxic gases (SO2, NO2 and NH3) on transition metal (Rh, Pd, Pt) modified Ti3C2F2 monolayer was explored using density functional theory calculations. To gain insights into the change of adsorption and gas-sensitive properties of Ti3C2F2 monolayer modified with metal atoms, the structures of metal modification and gas adsorption on Ti3C2F2, charge transfer, adsorption energy, band structure, state density and molecular orbitals were analyzed. It is found that transition metal atoms' modification on the substrate improves the conductivity of Ti3C2F2 monolayer. Moreover, the optimal structures for the modification of Ti₃C₂F₂ with Rh, Pd and Pt have been identified, with the binding energies of -2.614 eV, -0.819 eV and -1.411 eV guaranteeing the stability of the three structures during the adsorption process. The adsorption capacity of the original Ti3C2F2 for SO2, NO2 and NH3 is weak physical adsorption with adsorption energies in the range of -0.2 eV to -0.4 eV. Compared with the original Ti3C2F2, the adsorption efficiency of Rh-Ti3C2F2, Pd-Ti3C2F2 and Pt-Ti3C2F2 for SO2, NO2 and NH3 is significantly improved: the adsorption energies of Rh-Ti₃C₂F₂ for the three gases are -1.2 eV to -1.6 eV, Pd-Ti₃C₂F₂ are -1.6 eV to -1.8 eV, and Pt-Ti₃C₂F₂ are -1.1 eV to -2.2 eV, all reaching the level of chemical adsorption. In addition, the Pd-Ti3C2F2 monolayer exhibits high stability, and its structure remains unchanged after the adsorption of gases. Moreover, the analysis of the density of states indicates that Rh-Ti3C2F2 exhibits the most pronounced interaction with NH3 and the least significant interaction with NO2, whereas both Pd-Ti3C2F2 and Pt-Ti3C2F2 display the greatest interaction with NO2 and the weakest with NH3. Investigations into molecular orbitals suggest that Rh-Ti3C2F2's electrical conductivity when exposed to gas molecules is as follows: NH3 > SO2 > NO2, and the Eg(variation) values of the three gases are 2.96 %, 2.70 % and 2.16 % respectively. For Pd-Ti3C2F2, the conductivity influenced by gases is NO2 > NH3 = SO2 with the Eg(variation) values are 82.83 %, 1.26 % and 1.26 % respectively. Meanwhile, Pt-Ti3C2F2 exhibits conductivity changes in the sequence of NO2 > SO2 > NH3 when exposed to gas molecules where the Eg(variation) values are 24.54 %, 16.71 % and 8.62 % respectively. These findings offer a foundational theory for the fabrication of gas sensors utilizing Rh-Ti3C2F2, Pd-Ti3C2F2 and Pt-Ti3C2F2 for the monitoring of hazardous industrial gases.
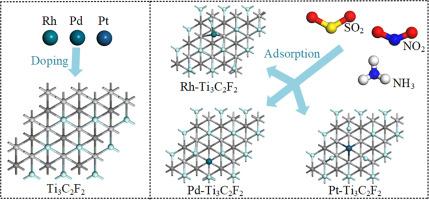
TM (Rh, Pd, Pt)修饰Ti3C2F2对SO2, NO2和NH3气体分子的吸附和气敏性能:DFT研究
利用密度泛函理论计算,研究了过渡金属(Rh, Pd, Pt)修饰的Ti3C2F2单层对工业有毒气体(SO2, NO2和NH3)的吸附和气敏性能。为了深入了解金属原子修饰Ti3C2F2单层膜的吸附和气敏性能的变化,分析了Ti3C2F2表面金属修饰和气体吸附的结构、电荷转移、吸附能、能带结构、态密度和分子轨道。发现过渡金属原子在基体上的修饰提高了Ti3C2F2单层的导电性。此外,还确定了Rh、Pd和Pt改性Ti₃C₂F₂的最佳结构,其结合能分别为-2.614 eV、-0.819 eV和-1.411 eV,保证了三种结构在吸附过程中的稳定性。原始Ti3C2F2对SO2、NO2和NH3的吸附能力为弱物理吸附,吸附能在-0.2 ~ -0.4 eV之间。与原Ti3C2F2相比,Rh-Ti3C2F2、Pd-Ti3C2F2和Pt-Ti3C2F2对SO2、NO2和NH3的吸附效率显著提高:Rh-Ti₃C₂F₂对3种气体的吸附能为-1.2 eV ~ -1.6 eV, Pd-Ti₃C₂F₂为-1.6 eV ~ -1.8 eV, Pt-Ti₃C₂F₂为-1.1 eV ~ -2.2 eV,均达到化学吸附水平。此外,Pd-Ti3C2F2单层具有较高的稳定性,吸附气体后其结构保持不变。态密度分析表明,Rh-Ti3C2F2与NH3的相互作用最显著,与NO2的相互作用最不显著,而Pd-Ti3C2F2和Pt-Ti3C2F2与NO2的相互作用最大,与NH3的相互作用最弱。对分子轨道的研究表明,Rh-Ti3C2F2暴露于气体分子时的电导率如下:NH3 >;二氧化硫比;三种气体的NO2和Eg(变异)值分别为2.96%、2.70%和2.16%。对于Pd-Ti3C2F2,受气体影响的电导率为NO2 >;NH3 = SO2, Eg(变异)值分别为82.83%、1.26%和1.26%。同时,Pt-Ti3C2F2呈现出NO2 >顺序的电导率变化;二氧化硫比;当NH3暴露于气体分子时,Eg(变异)值分别为24.54%、16.71%和8.62%。这些研究结果为利用Rh-Ti3C2F2、Pd-Ti3C2F2和Pt-Ti3C2F2制作用于工业有害气体监测的气体传感器提供了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


