Comprehensive proteomic, structural and functional analysis of cryoprotectant effects on marine mussel oocytes
IF 2.1
3区 生物学
Q2 BIOLOGY
引用次数: 0
Abstract
Cryopreservation is a valuable tool for preserving marine genetic diversity and supporting selective breeding in aquaculture. The blue mussel (Mytilus galloprovincialis) is an important species for the aquaculture industry and a useful model for studying cryopreservation in marine invertebrates. Declining mussel seed availability over the past decade, combined with the growing demand for year-round hatchery production, has increased interest in developing effective cryopreservation methods for gametes and embryos. However, cryopreservation remains challenging, particularly for marine invertebrate oocytes, as it requires balancing cryoprotectant cytotoxicity with sufficient cell protection during freezing and thawing. Moreover, the molecular, structural, and functional changes associated with oocyte cryopreservation in invertebrates are still poorly understood. This study examines the effects of two commonly used cryoprotectants, dimethyl sulfoxide (DMSO) and ethylene glycol (EG), on M. galloprovincialis oocytes under a monitored slow freezing (MSF) protocol. Using an integrative analytical approach combining proteomics, functional assessments, and structural analyses, we provide a comprehensive understanding of cryoprotectant-induced changes. Our findings reveal that cryoprotectants cause significant proteomic alterations, with DMSO having more pronounced effects. These alterations are associated with increased oxidative stress, an insufficient antioxidant response, and disruptions in meiosis restart mechanisms, ultimately delaying larval development. Cryoprotectant-induced oxidative stress may also weaken the oocyte membrane, leading to rupture during the MSF protocol and subsequent fertilisation failure post-thawing. These results provide evidence that EG is less cytotoxic than DMSO and suggest that supplementing cryoprotectants with antioxidants and membrane stabilizers could enhance success rates in the cryopreservation of oocytes from mussels and other marine invertebrate species.
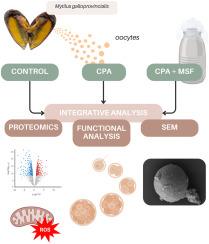
冷冻保护剂对海洋贻贝卵母细胞的蛋白质组学、结构和功能综合分析
低温保存是保护海洋遗传多样性和支持水产养殖选择性育种的重要手段。蓝贻贝(Mytilus galloprovincialis)是水产养殖业的重要物种,也是研究海洋无脊椎动物低温保存的有用模型。在过去的十年中,贻贝种子的可用性不断下降,加上对全年孵化场生产的需求不断增长,人们对开发有效的配子和胚胎冷冻保存方法的兴趣增加了。然而,冷冻保存仍然具有挑战性,特别是对于海洋无脊椎动物卵母细胞,因为它需要在冷冻和解冻期间平衡冷冻保护剂的细胞毒性和足够的细胞保护。此外,与无脊椎动物卵母细胞低温保存相关的分子、结构和功能变化仍然知之甚少。本研究考察了两种常用的冷冻保护剂,二甲基亚砜(DMSO)和乙二醇(EG)在监测慢速冷冻(MSF)方案下对加洛省m.s gallprovincialis卵母细胞的影响。使用结合蛋白质组学,功能评估和结构分析的综合分析方法,我们提供了对冷冻保护剂诱导的变化的全面了解。我们的研究结果表明,冷冻保护剂会引起显著的蛋白质组改变,其中DMSO的影响更为明显。这些改变与氧化应激增加、抗氧化反应不足以及减数分裂重启机制的破坏有关,最终延迟了幼虫的发育。低温保护剂诱导的氧化应激也可能削弱卵母细胞膜,导致在MSF方案中破裂和随后的解冻后受精失败。这些结果证明EG的细胞毒性低于DMSO,并表明在冷冻保护剂中添加抗氧化剂和膜稳定剂可以提高贻贝和其他海洋无脊椎动物卵母细胞的低温保存成功率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cryobiology
生物-生理学
CiteScore
5.40
自引率
7.40%
发文量
71
审稿时长
56 days
期刊介绍:
Cryobiology: International Journal of Low Temperature Biology and Medicine publishes research articles on all aspects of low temperature biology and medicine.
Research Areas include:
• Cryoprotective additives and their pharmacological actions
• Cryosurgery
• Freeze-drying
• Freezing
• Frost hardiness in plants
• Hibernation
• Hypothermia
• Medical applications of reduced temperature
• Perfusion of organs
• All pertinent methodologies
Cryobiology is the official journal of the Society for Cryobiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


