Electron pull-push modulation: Substrate controlled regioselectivity switch in 1,3-dipolar cycloaddition of isatin-derived azomethine ylides with chalcones
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A switch in regioselectivity has been achieved by modulating electron pull and push during the synthesis of spiropyrrolidine-oxindole analogs through 1,3-dipolar cycloaddition of isatin derived in-situ generated azomethine ylides with chalcones. Electron-withdrawing groups on ketone termini, along with neutral or electron-withdrawing groups on aldehyde termini, favored β-attack. In contrast, electron-donating groups on aldehyde termini switch the selectivity to α-attack. Structures of regioisomers have been determined by NMR, HRMS and SC-XRD.
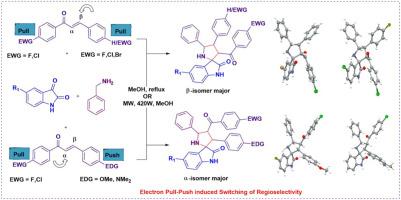
电子推拉调制:底物控制区域选择性开关在1,3-偶极环加成的isatin衍生亚甲基酰与查尔酮
通过调节1,3-偶极环加成,通过isatin衍生的原位生成的azomiine ylides与查尔酮合成螺旋吡咯烷-氧吲哚类似物,实现了区域选择性的切换。酮端上的吸电子基团和醛端上的中性或吸电子基团有利于β攻击。相反,醛端上的给电子基团将选择性转向α-攻击。区域异构体的结构已通过核磁共振、质谱和SC-XRD测定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


