Effects of Vutrisiran on Cardiac Function and Outcomes in Patients With Transthyretin Amyloidosis With Cardiomyopathy
IF 22.3
1区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
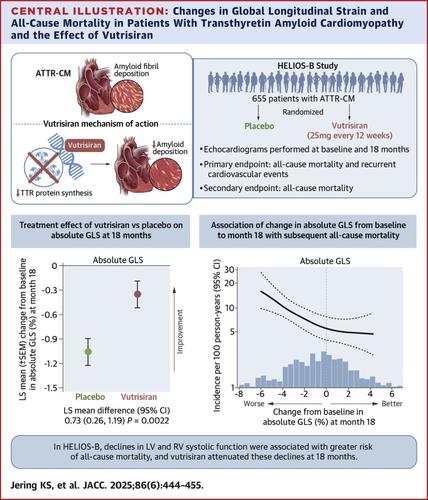
Transthyretin amyloid cardiomyopathy (ATTR-CM), caused by deposition of transthyretin amyloid fibrils in the heart, is associated with high morbidity and mortality. In HELIOS-B (A Study to Evaluate Vutrisiran in Patients With Transthyretin Amyloidosis With Cardiomyopathy), the RNA interference therapeutic agent vutrisiran reduced rates of the primary composite outcome of all-cause death and recurrent cardiovascular events among patients with ATTR-CM and had beneficial effects on cardiac structure and function over 30 months.
Objectives
The purpose of this study was to investigate associations of echocardiographic measures of cardiac structure and function with the primary outcome and to assess whether favorable changes in cardiac structure and function with vutrisiran were associated with improvements in outcomes.
Methods
HELIOS-B randomized 655 patients with ATTR-CM to vutrisiran (25 mg subcutaneously every 12 weeks) or placebo. Echocardiograms were performed at baseline and months 12, 18, 24, and 30. Associations of baseline echocardiographic parameters with the primary outcome were analyzed using modified Andersen-Gill models adjusted for age, sex, ATTR disease type, and National Amyloidosis Centre stage, and stratified by baseline tafamidis use and treatment assignment. Changes in cardiac function from baseline to month 18 were compared between treatment arms and related to outcomes in landmark analyses.
Results
Among the 654 participants with available echocardiographic data (median age 77 years, 93% male, 88% wild-type transthyretin), baseline left and right ventricular systolic and diastolic function were independently associated with the primary outcome (HR per unit increase, left ventricular ejection fraction, 0.90 per 5% increase, 95% CI: 0.86-0.95; absolute global longitudinal strain, 0.92 per 1% increase, 95% CI: 0.89-0.96; tricuspid annular systolic myocardial velocity, 0.94 per 1-cm/s increase, 95% CI: 0.90-0.98; average E/e’, 1.03 per 1-U increase, 95% CI: 1.01-1.04). At 18 months, vutrisiran attenuated declines in left ventricular and right ventricular systolic function (least squares mean difference: left ventricular ejection fraction, 1.6%, 95% CI: 0.1-3.2; absolute global longitudinal strain, 0.7%, 95% CI: 0.3-1.2; tricuspid annular systolic myocardial velocity, 0.5 cm/s, 95% CI: 0.1-0.9). Worsening in these parameters at 18 months was associated with a heightened risk of the primary outcome.
Conclusions
Echocardiographic measures of biventricular systolic and diastolic function provide important prognostic information beyond National Amyloidosis Centre stage in patients with ATTR-CM. Vutrisiran improved diastolic function and attenuated declines in left ventricular and right ventricular systolic function over 18 months. The benefits on cardiac function with vutrisiran may partly underlie its beneficial effects on clinical outcomes.
伏曲西兰对转甲状腺素淀粉样变合并心肌病患者心功能及预后的影响
转甲状腺蛋白淀粉样心肌病(atr - cm)是由转甲状腺蛋白淀粉样原纤维沉积在心脏引起的,具有很高的发病率和死亡率。在HELIOS-B(一项评估vtrisiran治疗经甲状腺蛋白淀粉样变性合并心肌病患者的研究)中,RNA干扰治疗药物vtrisiran降低了atr - cm患者全因死亡和心血管事件复发的主要复合结局率,并在30个月内对心脏结构和功能产生有益影响。目的:本研究的目的是调查心脏结构和功能的超声心动图测量与主要结局的关系,并评估心脏结构和功能的有利改变是否与乌崔西兰治疗的预后改善有关。方法shelios - b将655例atr - cm患者随机分组,给予乌崔西兰(每12周皮下注射25 mg)或安慰剂。在基线和12、18、24和30个月进行超声心动图检查。使用经年龄、性别、ATTR疾病类型和国家淀粉样变性中心分期调整的改进Andersen-Gill模型分析基线超声心动图参数与主要结局的关系,并根据基线他法米迪的使用和治疗分配进行分层。从基线到18个月的心功能变化在治疗组之间进行比较,并与里程碑分析的相关结果进行比较。结果在654名有超声心动图数据的参与者中(中位年龄77岁,93%为男性,88%为野生型甲状腺素转移),基线左、右心室收缩和舒张功能与主要结局独立相关(HR每单位增加,左心室射血分数,0.90每增加5%,95% CI: 0.86-0.95;绝对全球纵向应变,每增加1% 0.92,95% CI: 0.89-0.96;三尖瓣环状收缩心肌速度,每增加1 cm/s 0.94, 95% CI: 0.90-0.98;平均E/ E′,每增加1 u增加1.03,95% CI: 1.01-1.04)。18个月时,vutrisiran减轻了左室和右室收缩功能的下降(最小二乘平均差:左室射血分数,1.6%,95% CI: 0.1-3.2;全球绝对纵向应变,0.7%,95% CI: 0.3-1.2;三尖瓣环状收缩心肌速度,0.5 cm/s, 95% CI: 0.1-0.9)。这些参数在18个月时恶化与主要结局的高风险相关。结论超声心动图测量双心室收缩和舒张功能对atr - cm患者的预后提供了重要的信息。在18个月的时间里,Vutrisiran改善了左心室和右心室的舒张功能,减轻了左心室和右心室收缩功能的下降。乌崔西兰对心功能的益处可能部分地解释了其对临床结果的有益影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
42.70
自引率
3.30%
发文量
5097
审稿时长
2-4 weeks
期刊介绍:
The Journal of the American College of Cardiology (JACC) publishes peer-reviewed articles highlighting all aspects of cardiovascular disease, including original clinical studies, experimental investigations with clear clinical relevance, state-of-the-art papers and viewpoints.
Content Profile:
-Original Investigations
-JACC State-of-the-Art Reviews
-JACC Review Topics of the Week
-Guidelines & Clinical Documents
-JACC Guideline Comparisons
-JACC Scientific Expert Panels
-Cardiovascular Medicine & Society
-Editorial Comments (accompanying every Original Investigation)
-Research Letters
-Fellows-in-Training/Early Career Professional Pages
-Editor’s Pages from the Editor-in-Chief or other invited thought leaders

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


