20-Hydroxyecdysone regulates the expression of antimicrobial peptides through dorsal and relish in Helicoverpa armigera
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
20-hydroxyecdysone (20E) plays a key role in insect development, not only regulating molting and metamorphosis but also participating in the innate immune responses. The regulation of immunity by 20E varies among different insect species. The pathway and mechanism of 20E regulates immunity in the cotton bollworm (Helicoverpa armigera) remain unclear. In this study, we confirmed that the enhanced immunity is regulated by 20E in H. armigera. 20E upregulates the expression of antimicrobial peptides (AMPs). Knockdown of Dorsal suppressed the 20E-induced expression of AMPs, while knockdown of Relish similarly inhibited the expression of AMPs except Moricin. Overexpression of Dorsal promotes its nuclear translocation and activates the expression of AMPs. However, overexpression of Cactus (IκB-like protein, the inhibitor of NFκB) isoform A binds to Dorsal and inhibits its nuclear translocation. 20E upregulates Dorsal expression via direct EcR mediation and facilitates Dorsal release through phosphorylation of Cactus A. We also report spontaneous activation of HaRelish through auto-proteolytic cleavage, liberating its N-terminal domain (Rel-N). Nuclear Rel-N interacts with Dorsal to co-regulate AMP transcription. These data indicate that 20E induces the expression of AMPs in H. armigera through activation of Dorsal and Relish, thereby enhancing immunity during the metamorphosis stage.
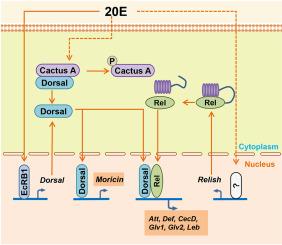
20-羟基蜕皮激素通过棉铃虫背侧和津津乐道调节抗菌肽的表达。
20-羟基蜕皮激素(20-hydroxyecdysone, 20E)在昆虫发育过程中起着关键作用,不仅调节昆虫的蜕皮和变态,还参与昆虫的先天免疫反应。20E对不同昆虫的免疫调节是不同的。20E调节棉铃虫(Helicoverpa armigera)免疫的途径和机制尚不清楚。在本研究中,我们证实了这种增强的免疫是由20E调节的。20E上调抗菌肽(AMPs)的表达。低敲Dorsal抑制了20e诱导的AMPs的表达,而低敲enjoy同样抑制了除Moricin外的AMPs的表达。Dorsal的过表达促进其核易位,激活amp的表达。然而,过表达Cactus (i - κ b样蛋白,nf - κ b抑制剂)异构体A与背侧结合并抑制其核易位。20E通过直接EcR介导上调背侧表达,并通过Cactus a的磷酸化促进背侧释放。我们还报道了ha回味蛋白通过自身蛋白水解裂解而自发激活,释放其n端结构域(Rel-N)。核Rel-N与Dorsal相互作用,共同调节AMP转录。这些数据表明,20E通过激活Dorsal和回味蛋白诱导棉铃虫AMPs的表达,从而增强棉铃虫变态期的免疫力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


