Fisetin alleviates Aβ-induced neuronal cell ferroptosis by regulating Sirt6-mediated deacetylation modification
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Background
Ferroptosis contributes to Alzheimer's disease (AD) pathology. Fisetin, a flavonoid with neuroprotective properties, has shown potential in reducing oxidative stress and inflammation. This study investigates the protective effects and underlying molecular pathways of fisetin against amyloid-β (Aβ)-induced ferroptosis in neuronal cells.
Methods
HT22 mouse hippocampal neuronal cells were pre-incubated with fisetin before exposure to amyloid-β (Aβ). Cell viability was evaluated by Cell Counting Kit-8 (CCK-8) assay, and lipid peroxidation was measured with the C11-BODIPY probe. Protein expression patterns were determined via Western blot analysis. Co-immunoprecipitation (CoIP) and ubiquitination assays were used to explore the interactions between Sirtuin 6 (Sirt6), nuclear factor erythroid 2-related factor 2 (Nrf2), and kelch-like ECH-associated protein 1 (Keap1).
Results
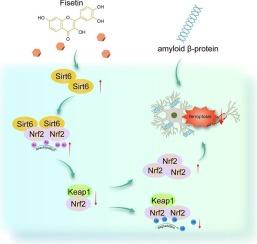
Fisetin pre-treatment significantly alleviated Aβ-induced ferroptosis in HT22 cells. Sirt6 upregulation was observed following fisetin treatment, and silencing Sirt6 reversed its protective effects on Aβ-induced neuronal cell ferroptosis. Mechanistically, Sirt6 deacetylated Nrf2 and enhanced its stability by preventing its degradation through Keap1-mediated ubiquitination. As expected, fisetin alleviated Aβ-induced ferroptosis in neuronal cells through activation of the Sirt6/Nrf2 axis.
Conclusion
Fisetin alleviated Aβ-induced neuronal cell ferroptosis by activating the Sirt6/Nrf2 axis. These results underscore fisetin's therapeutic potential for AD by targeting ferroptosis, providing a new strategy for disease intervention.
非西汀通过调节sirt6介导的去乙酰化修饰减轻a β诱导的神经元细胞铁凋亡
背景:铁下垂与阿尔茨海默病(AD)病理有关。非瑟酮是一种具有神经保护作用的类黄酮,已显示出减少氧化应激和炎症的潜力。本研究探讨了非瑟酮对淀粉样蛋白-β (Aβ)诱导的神经元细胞铁下垂的保护作用和潜在的分子途径。方法将sht22小鼠海马神经元细胞用非西汀预孵育后再暴露于淀粉样蛋白-β (Aβ)。细胞计数试剂盒-8 (CCK-8)检测细胞活力,C11-BODIPY探针检测脂质过氧化。Western blot检测蛋白表达谱。采用共免疫沉淀法(CoIP)和泛素化法研究Sirtuin 6 (Sirt6)、核因子红细胞2相关因子2 (Nrf2)和kelch样ech相关蛋白1 (Keap1)之间的相互作用。结果非西汀预处理可显著减轻a β诱导的HT22细胞铁下垂。非瑟酮治疗后Sirt6上调,沉默Sirt6逆转了其对a β诱导的神经元细胞铁凋亡的保护作用。在机制上,Sirt6通过keap1介导的泛素化阻止Nrf2降解,从而使Nrf2去乙酰化并增强其稳定性。正如预期的那样,非瑟酮通过激活Sirt6/Nrf2轴减轻了a β诱导的神经元细胞铁凋亡。结论非西汀通过激活Sirt6/Nrf2轴减轻a β诱导的神经元细胞铁下垂。这些结果强调了非塞汀通过靶向铁下垂治疗AD的潜力,为疾病干预提供了新的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


