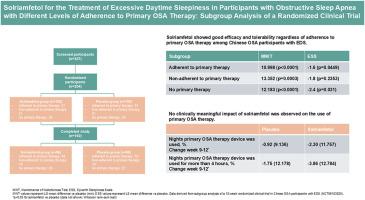
Solriamfetol for the treatment of excessive daytime sleepiness in participants with obstructive sleep apnea with different levels of adherence to primary OSA therapy: Subgroup analysis of a randomized clinical trial
IF 3.4
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Background
Solriamfetol is a dopamine and norepinephrine reuptake inhibitor (DNRI) indicated for the treatment of excessive daytime sleepiness (EDS) associated with obstructive sleep apnea (OSA). The current study aims to evaluate effects of solriamfetol among participants with different adherence to primary OSA therapy using data from a randomized clinical trial conducted among Chinese participants.
Methods
Participants were 1:1 randomized to placebo or solriamfetol (up to 150 mg/day) for 12 weeks (stratified by adherence to primary OSA therapy). The coprimary endpoints were change from baseline in mean sleep latency of maintenance of wakefulness test (MWT) and Epworth Sleepiness Scale (ESS) at week 12 in the full analysis set. Use of primary OSA therapy and safety were also evaluated.
Results
At baseline, of all participants, around 50 % were adherent, 20 % were non-adherent and 30 % were not on primary OSA therapy, respectively. In all subgroups, solriamfetol treatment was associated with significant or numerical improvement in MWT sleep latency or ESS (LS mean difference vs. placebo, p < 0.05 except ESS in non-adherence to primary therapy subgroup). Use of primary OSA therapy was stable throughout the 12-week study. Solriamfetol was well tolerated and the most common TEAEs included metabolism and nutrition disorders, upper respiratory tract infection, dizziness, hypertension and elevated blood creatine phosphokinase.
Conclusion
In the subgroup analysis of a randomized clinical trial in Chinese OSA participants with EDS, solriamfetol was effective and well tolerated regardless of adherence to primary OSA therapy. No clinically meaningful impact of solriamfetol on the use of primary OSA therapy was found.
索利氨酚治疗阻塞性睡眠呼吸暂停患者日间过度嗜睡的疗效:一项随机临床试验亚组分析
索利氨酚是一种多巴胺和去甲肾上腺素再摄取抑制剂(DNRI),用于治疗与阻塞性睡眠呼吸暂停(OSA)相关的日间过度嗜睡(EDS)。本研究旨在利用一项随机临床试验的数据,评估索利氨酚对不同坚持原发性OSA治疗的受试者的影响。方法参与者按1:1随机分为安慰剂组或索利氨酚组(最高150mg /天),持续12周(按坚持原发性OSA治疗分层)。主要终点是在完整分析集的第12周,维持清醒测试(MWT)和Epworth嗜睡量表(ESS)的平均睡眠潜伏期较基线的变化。对原发性阻塞性睡眠呼吸暂停治疗的使用和安全性也进行了评估。结果基线时,在所有参与者中,约50%的患者坚持接受原发性OSA治疗,20%的患者不坚持,30%的患者未接受原发性OSA治疗。在所有亚组中,索利氨酚治疗与MWT睡眠潜伏期或ESS的显著或数值改善相关(LS与安慰剂的平均差异,p <;除ESS外,未依从主要治疗亚组0.05)。在整个12周的研究中,原发性OSA治疗的使用是稳定的。索利氨酚耐受性良好,最常见的teae包括代谢和营养障碍、上呼吸道感染、头晕、高血压和血肌酸磷酸激酶升高。结论在一项针对中国OSA患者EDS的随机临床试验的亚组分析中,无论是否坚持最初的OSA治疗,索利氨酚都是有效且耐受性良好的。未发现索利氨酚对OSA原发性治疗的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sleep medicine
医学-临床神经学
CiteScore
8.40
自引率
6.20%
发文量
1060
审稿时长
49 days
期刊介绍:
Sleep Medicine aims to be a journal no one involved in clinical sleep medicine can do without.
A journal primarily focussing on the human aspects of sleep, integrating the various disciplines that are involved in sleep medicine: neurology, clinical neurophysiology, internal medicine (particularly pulmonology and cardiology), psychology, psychiatry, sleep technology, pediatrics, neurosurgery, otorhinolaryngology, and dentistry.
The journal publishes the following types of articles: Reviews (also intended as a way to bridge the gap between basic sleep research and clinical relevance); Original Research Articles; Full-length articles; Brief communications; Controversies; Case reports; Letters to the Editor; Journal search and commentaries; Book reviews; Meeting announcements; Listing of relevant organisations plus web sites.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


