Cardiomyocyte Janus kinase 1 (JAK1) signaling is required for cardiac homeostasis and cytokine-dependent activation of STAT3
IF 4.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Despite the essential role of inflammation in the pathogenesis of heart failure and other chronic cardiovascular diseases, how cardiomyocytes sense and respond to the inflammatory milieu is not well understood. Cytokine receptors respond to circulating glycoprotein 130 (gp130) family cytokines, such as interleukin-6 (IL-6) and oncostatin M (OSM), by signaling through Janus kinases (JAK) to ultimately elicit phosphorylation-dependent nuclear translocation and transcriptional activity of signal transducer and activator of transcription (STAT) proteins. JAK1 is particularly important for STAT3-dependent cytokine production and macrophage recruitment by cardiomyocytes and STAT3 promotes cardiac hypertrophy and remodeling in response to pressure overload or angiotensin-II but is protective during ischemic injury. However, the roles of JAK1 signaling in cardiac homeostasis and cardiomyocyte cytokine sensing and responsivity remain unclear. To assess the functions of JAK1 in cardiac physiology, we generated mice with cardiomyocyte-specific deletion of JAK1 and evaluated cardiac structure and function, myocardial remodeling, and intracellular signal transduction. Loss of JAK1 in cardiomyocytes results in dilated cardiomyopathy by 6 months of age, indicating cytokine receptor signaling through JAK1 is essential for cardiac physiology. Cardiomyopathy in aged mice lacking cardiomyocyte JAK1 was characterized by substantial myocardial fibrosis. Transcriptomics and gene expression analyses identified JAK1-dependent cytokine-inducible target genes in adult cardiomyocytes as putative effectors of JAK1-STAT3 in the cardiac stress response. JAK1-deficient adult cardiomyocytes were resistant to phosphorylation and nuclear translocation of STAT3 and transcriptional reprogramming in response to OSM. Collectively these data indicate cardiomyocyte JAK1 kinase activity is required for proper cardiac maturation and homeostasis and is indispensable for STAT3 activation and transcriptional responses to OSM.
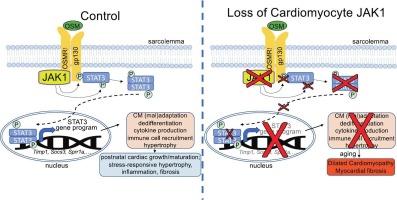
心肌细胞Janus激酶1 (JAK1)信号是心脏稳态和STAT3细胞因子依赖性激活所必需的。
尽管炎症在心力衰竭和其他慢性心血管疾病的发病机制中起着至关重要的作用,但心肌细胞如何感知和响应炎症环境尚不清楚。细胞因子受体响应循环糖蛋白130 (gp130)家族细胞因子,如白介素-6 (IL-6)和肿瘤抑制素M (OSM),通过Janus激酶(JAK)发出信号,最终引发磷酸化依赖的核易位和信号传导和转录激活因子(STAT)蛋白的转录活性。JAK1对于STAT3依赖性细胞因子的产生和心肌细胞的巨噬细胞募集尤为重要,STAT3在压力过载或血管紧张素- ii的反应中促进心脏肥大和重塑,但在缺血性损伤时具有保护作用。然而,JAK1信号在心脏稳态和心肌细胞细胞因子感知和反应中的作用尚不清楚。为了评估JAK1在心脏生理学中的功能,我们制造了心肌细胞特异性缺失JAK1的小鼠,并评估了心脏结构和功能、心肌重塑和细胞内信号转导。心肌细胞中JAK1的缺失导致6 月龄时扩张型心肌病,表明细胞因子受体通过JAK1信号传导对心脏生理至关重要。心肌细胞JAK1缺失的老年小鼠心肌病以心肌纤维化为特征。转录组学和基因表达分析发现,成人心肌细胞中jak1依赖性细胞因子诱导靶基因可能是心脏应激反应中JAK1-STAT3的效应因子。在OSM的作用下,jak1缺陷的成人心肌细胞对STAT3的磷酸化和核易位以及转录重编程具有抗性。总的来说,这些数据表明心肌细胞JAK1激酶活性是心脏成熟和稳态所必需的,也是STAT3激活和OSM转录反应所必需的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


