Insight in the role of resistant starch and Eudragit S 100 in a coating for controlled release in the colonic region
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-07-29
DOI:10.1016/j.ejpb.2025.114818
引用次数: 0
Abstract
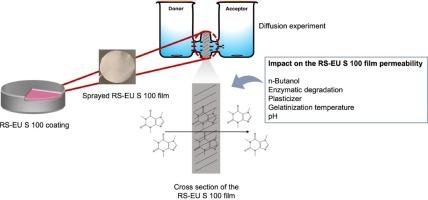
A dual trigger coating was designed by Ibekwe et al. to achieve site-specific delivery of therapeutics to the colon. It consists essentially of Eudragit® S 100 (EU S 100), a polymer that dissolves above pH 7, and resistant starch, which can only be degraded by enzymes produced by colonic bacteria. Both components should maintain the integrity of the coating until reaching the colonic region and ensure drug release when reaching it. The contribution of these components was investigated in the present study by permeability testing and enzymatic degradation testing of free polymer films produced using an in-house built spraying device.
Diffusion testing of EU S 100 films showed no impact of the pH (2, 4, 6) and plasticizer levels (25, 30, 35 % w/w triethyl citrate (TEC)) on the permeability of the films. The permeability of the resistant starch-Eudragit® S 100 (RS-EU S 100) films was also not affected by the pH, however, the permeability coefficient of films containing 35 % w/w TEC was significantly higher for all pH levels. Incubating the RS-EU S 100 films with enzyme resulted in a higher permeability for films with 25 % w/w TEC, yet the presence of enzyme had no effect on the permeability of 35 % w/w TEC films. For the 25 % w/w TEC films, even if EU S 100 does not dissolve (pH < 7), the resistant starch in the RS-EU S 100 film can be enzymatically degraded.
Using n-butanol in the preparation process of the RS-EU S 100 films resulted in a lower permeability coefficient compared to the RS-EU S 100 films without n-butanol for all pH and plasticizer levels. n-Butanol forms a V-type complex with amylose, which makes the films less susceptible to enzymatic degradation.
The permeability and susceptibility to enzymatic degradation of the RS-EU S 100 coating can be adapted by altering the amount of plasticizer and the use of n-butanol during the production process.
抗性淀粉和Eudragit s100在结肠区域控释涂层中的作用。
Ibekwe等人设计了一种双触发涂层,以实现治疗药物到结肠的部位特异性递送。它主要由Eudragit®S 100 (EU S 100)组成,这是一种溶解在pH值7以上的聚合物,以及只能由结肠细菌产生的酶降解的抗性淀粉。这两种成分都应保持涂层的完整性,直到到达结肠区域,并确保到达时药物释放。在本研究中,通过渗透性测试和酶降解测试,研究了这些成分的贡献,这些测试是用内部建造的喷涂装置生产的自由聚合物薄膜。EU s100薄膜的扩散试验表明,pH(2、4、6)和增塑剂浓度(25、30、35 % w/w柠檬酸三乙酯(TEC))对膜的渗透性没有影响。抗性淀粉- eudragit®S 100 (RS-EU S 100)薄膜的渗透性也不受pH值的影响,但在所有pH值水平下,含有35% % w/w TEC的薄膜的渗透系数都显著较高。酶对25 % w/w TEC的RS-EU S 100膜具有较高的通透性,而对35 % w/w TEC膜的通透性没有影响。对于25 % w/w的TEC薄膜,即使EU s100不溶解(pH
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


