TMBIM4 affects left-right patterning via pluripotency exit during gastrulation
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
Congenital heart disease (CHD) is the most prevalent congenital defect, but its underlying genetic and developmental mechanisms remain incompletely understood. Transmembrane BAX inhibitor motif-containing protein 4 (TMBIM4) has emerged as a candidate gene from genomic studies in CHD patients. Patients with deleterious genetic variation in TMBIM4 can exhibit cardiac heterotaxy, a type of left-right (LR) patterning defect characterized by abnormal cardiac asymmetry. Using Xenopus tropicalis, we investigated tmbim4's developmental roles and identified its critical function in LR patterning. tmbim4 depletion in Xenopus produced cardiac asymmetry defects which could be rescued by human and viral orthologs of the protein, reflecting remarkable evolutionary conservation. We identified gastrulation as a critical window for tmbim4 function. tmbim4 depletion impairs gastrulation, leading to abnormal pluripotency marker expression and delayed pluripotency exit. TMBIM4's underlying function is as a putative ion channel, and ion channels are emerging as key regulators of LR patterning and cell fate determination. Using sharp electrodes to measure membrane potential (Vm), tmbim4 depletion depolarized affected embryos. The application of choline, which we have previously shown recues depolarization of Xenopus embryos, rescued the gastrulation defects and pluripotency in tmbim4 depleted embryos. Interestingly, TMBIM4 has previously been localized to the Golgi, and therefore how it might affect Vm was unclear. We find evidence that TMBIM4 localizes to the plasma membrane as well as the Golgi suggesting that it may directly act to establish cellular Vm. Our results establish tmbim4 as a plausible CHD gene and offer the first study of tmbim4 in a developmental context.
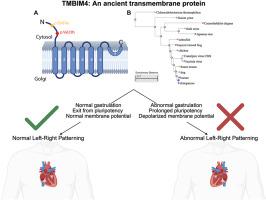
TMBIM4在原肠胚形成过程中通过多能性退出影响左右模式。
先天性心脏病(CHD)是最常见的先天性缺陷,但其潜在的遗传和发育机制尚不完全清楚。跨膜BAX抑制剂基序-含蛋白4 (TMBIM4)已成为冠心病患者基因组研究的候选基因。TMBIM4有害遗传变异的患者可表现为心脏异位,这是一种以心脏异常不对称为特征的左-右(LR)模式缺陷。以热带非洲爪蟾为研究对象,研究了tmbim4在热带爪蟾发育中的作用,并确定了其在热带爪蟾LR模式中的关键功能。非洲爪蟾的tmbim4缺失会产生心脏不对称缺陷,这种缺陷可以通过该蛋白的人类和病毒同源物来修复,反映出显著的进化保守性。我们发现原肠形成是tmbim4功能的一个关键窗口。Tmbim4缺失损害原肠胚形成,导致多能性标志物表达异常和多能性退出延迟。TMBIM4的潜在功能是作为一个假定的离子通道,离子通道正在成为LR模式和细胞命运决定的关键调节因子。利用尖锐电极测量膜电位(Vm), tmbim4耗竭去极化影响胚胎。胆碱的应用(我们之前已经证明胆碱可以减少爪蟾胚胎的去极化)挽救了tmbim4缺失胚胎的原肠胚发育缺陷和多能性。有趣的是,TMBIM4先前已经定位于高尔基体,因此它如何影响Vm尚不清楚。我们发现TMBIM4定位于质膜和高尔基体的证据表明,它可能直接作用于细胞Vm的建立。我们的研究结果证实了tmbim4是一种可能的冠心病基因,并首次在发育背景下研究了tmbim4。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


