Non-invasive Diagnosis of Antinephrin–Associated Podocytopathy
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
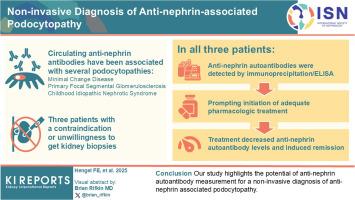
Circulating autoantibodies against the podocyte surface protein nephrin have recently been described in patients with podocytopathies, that is, minimal change disease, primary focal segmental glomerulosclerosis, and childhood idiopathic nephrotic syndrome. Their high specificity for podocytopathies in combination with a strong correlation with disease activity hold the potential for a non-invasive diagnosis, but prospective data are lacking.
Methods
Here, we describe 3 patients with contraindications or unwillingness for a kidney biopsy, hampering a timely histological diagnosis and choice of appropriate therapy.
Results
In all patients, antinephrin autoantibodies were detected by quantitative immunoprecipitation, prompting the initiation of adequate treatment. These interventions induced a decrease in antinephrin autoantibody levels and clinical remission.
Conclusion
Our study highlights the potential of antinephrin autoantibody measurement for a noninvasive diagnosis of antinephrin-associated podocytopathy.
抗肾上腺素相关性足细胞病的无创诊断
针对足细胞表面蛋白nephrin的循环自身抗体最近在足细胞病变(即微小改变疾病、原发性局灶节段性肾小球硬化和儿童特发性肾病综合征)患者中被描述。它们对足细胞病变的高特异性以及与疾病活动的强相关性具有非侵入性诊断的潜力,但缺乏前瞻性数据。方法在此,我们描述了3例有禁忌症或不愿进行肾活检的患者,妨碍了及时的组织学诊断和选择适当的治疗方法。结果所有患者均通过定量免疫沉淀检测到抗肾上腺素自身抗体,提示开始适当的治疗。这些干预导致抗肾上腺素自身抗体水平下降和临床缓解。结论:本研究强调了抗肾上腺素自身抗体检测在无创诊断抗肾上腺素相关足细胞病中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


