Long-term Outcome of Adult Patients With Membranous Nephropathy Treated With Rituximab
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
Rituximab (RTX) therapy has become the standard of care for treatment of membranous nephropathy (MN). However, data on hard outcomes such as end-stage kidney disease (ESKD) and loss of estimated glomerular filtration rate (eGFR), are lacking.
Methods
This was a retrospective study on all patients with MN treated with RTX between January 2000 and December 2022. The primary outcomes were ESKD and eGFR loss > 50%. Clinical outcomes were complete remission (CR), partial remission (PR) (reduction in baseline proteinuria ≥ 50% and proteinuria ≤ 3.5 g/24 h), and immunological remission (IR) (serum antiphospholipase A receptor antibody [PLA2R-Ab] depletion).
Results
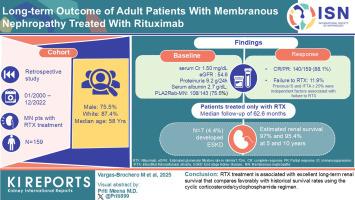
A total of 159 patients were included (75.5% male, 87.4% White, median age: 58 years); 52.8% had previous immunosuppression (IS). Baseline serum creatinine was 1.50 (1.1–1.9) mg/dl, eGFR was 54.6 (37.4–72.5) ml/min per 1.73 m2, proteinuria was 9.2 (6.7–11.9) g/24 h, and serum albumin was 2.7 (2.2–3.2) g/dl; Of the patients, 108 (75.5%) had PLA2R-Ab–associated MN (PLA2R-MN); and 140 of 159 (88.1%) attained CR or PR. Median (interquartile range [IQR]) time to CR and PR were 22.6 (15.5–37.4) and 6.8 (3.6–12.1) months, respectively. Failure to respond to RTX was observed in 11.9% of patients. Previous IS and interstitial fibrosis/tubular atrophy (IFTA) ≥ 25% were independent factors associated with failure to respond to RTX. Patients treated only with RTX with a median follow-up of 62.6 months; 7 of 159 (4.4%) developed ESKD with an estimated renal survival of 97% (95% confidence interval [CI]: 94%–100%) and 95.4% (95% CI: 91.2%–99%) at 5 and 10 years, respectively.
Conclusion
RTX treatment is associated with excellent long-term renal survival that compares favorably with historical survival rates using the cyclic corticosteroids/cyclophosphamide regimen.
利妥昔单抗治疗膜性肾病成人患者的长期疗效
利妥昔单抗(RTX)治疗已成为膜性肾病(MN)治疗的标准护理。然而,缺乏诸如终末期肾病(ESKD)和估计肾小球滤过率(eGFR)损失等硬结局的数据。方法回顾性研究2000年1月至2022年12月期间接受RTX治疗的所有MN患者。主要结局为ESKD和eGFR损失;50%。临床结果为完全缓解(CR)、部分缓解(PR)(基线蛋白尿减少≥50%,蛋白尿≤3.5 g/24 h)和免疫缓解(IR)(血清抗磷脂酶A受体抗体[PLA2R-Ab]消耗)。结果共纳入159例患者,其中男性75.5%,白人87.4%,中位年龄58岁;52.8%既往有免疫抑制(IS)。基线血清肌酐为1.50 (1.1-1.9)mg/dl, eGFR为54.6 (37.4-72.5)ml/min / 1.73 m2,蛋白尿为9.2 (6.7-11.9)g/24 h,血清白蛋白为2.7 (2.2-3.2)g/dl;在患者中,108例(75.5%)患有pla2r - ab相关性MN (PLA2R-MN);159例患者中有140例(88.1%)达到CR或PR。达到CR和PR的中位时间(四分位间距[IQR])分别为22.6(15.5-37.4)和6.8(3.6-12.1)个月。11.9%的患者对RTX无效。既往IS和间质纤维化/管状萎缩(IFTA)≥25%是与RTX无效相关的独立因素。仅接受RTX治疗的患者中位随访时间为62.6个月;159例患者中有7例(4.4%)发展为ESKD,估计5年和10年肾脏存活率分别为97%(95%可信区间[CI]: 94%-100%)和95.4% (95% CI: 91.2%-99%)。结论rtx治疗与环皮质激素/环磷酰胺方案的历史生存率相比具有良好的长期肾生存相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


