Chronic Changes on Kidney Histology by a Multiclass Artificial Intelligence Model
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
Chronic changes in kidney histology are often approximated by using human vision but with limited accuracy.
Methods
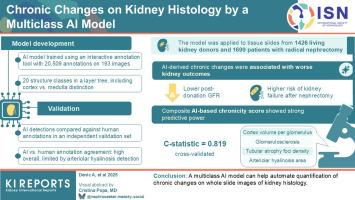
An interactive annotation tool trained an artificial intelligence (AI) model for segmenting structures on whole slide images (WSIs) of kidney tissue. A total of 20,509 annotations trained the AI model with 20 classes of structures, including separate detection of cortex from medulla. We compared the AI model detections with human-based annotations in an independent validation set. The AI model was then applied to 1426 donors and 1699 patients with renal tumor to calculate chronic changes as defined by measures of nephron size (glomerular volume, cortex volume per glomerulus, and mean tubular areas) and nephrosclerosis (globally sclerotic glomeruli, increased interstitium, increased tubular atrophy (TA), arteriolar hyalinosis (AH), and artery luminal stenosis from intimal thickening). We then assessed whether chronic kidney disease (CKD) outcomes were associated with these chronic changes.
Results
During the AI model validation step, the agreement between the AI detections and human annotations was similar to the agreement between human pairs, except that the AI model showed less agreement with AH. Chronic changes calculated solely from AI-based detections associated with low glomerular filtration rate (GFR) during follow-up after kidney donation and with kidney failure after a radical nephrectomy for tumor. A chronicity score based on AI detections was calculated from cortex per glomerulus, percent glomerulosclerosis, TA foci density, and mean area of AH lesions and showed good prognostic discrimination for kidney failure (cross-validation C-statistic = 0.819).
Conclusion
A multiclass AI model can help automate quantification of chronic changes on WSIs of kidney histology.
多类别人工智能模型对肾脏组织学慢性变化的影响
肾脏组织学的慢性变化通常用人类视觉来近似,但精度有限。方法利用交互式注释工具训练人工智能(AI)模型,对肾脏组织全切片图像(wsi)进行结构分割。总共有20509个注释训练了20类结构的AI模型,包括皮层和髓质的单独检测。我们在一个独立的验证集中比较了人工智能模型检测与基于人类的注释。然后将AI模型应用于1426名供体和1699名肾肿瘤患者,通过测量肾单位大小(肾小球体积、每肾小球皮质体积和平均肾小管面积)和肾硬化(肾小球整体硬化、间质增加、肾小管萎缩(TA)增加、小动脉透明质症(AH)和内膜增厚导致的动脉管腔狭窄)来计算慢性变化。然后我们评估慢性肾脏疾病(CKD)结局是否与这些慢性变化相关。结果在人工智能模型验证阶段,人工智能检测与人类注释之间的一致性与人类配对之间的一致性相似,只是人工智能模型与AH的一致性较低。仅通过人工智能检测计算的慢性变化与肾捐赠后随访期间肾小球滤过率(GFR)低和肿瘤根治性肾切除术后肾衰竭相关。基于AI检测计算每肾小球皮质、肾小球硬化百分比、TA病灶密度和AH病变平均面积的慢性评分,显示出对肾衰竭的良好预后鉴别(交叉验证c -统计量= 0.819)。结论多级人工智能模型可自动量化肾组织wsi的慢性变化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


