Large π-π interconnected guanidine based high-energy compounds and their trigger bonds
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
TNB, triazole, tetrazole, furoxan, guanidine, etc. are the basic building blocks for building high-energy compounds. Compounds with different structures and properties can be obtained by combining them in different ways (through atomic or group bridging, spiking, fusing, etc.). How to measure the effectiveness of their connection is what we must consider when designing high-energy compounds. Guanidine is Y-aromatic, and it is connected with other single or several aromatic rings to form large π-π interconnected compounds. The large π-π separation energy can measure the additional stabilization energy of large π-π interconnected structures due to electron delocalization, which is a new index of aromatic extension or aromaticity of compounds. It is also a major index of molecular deformability of high-energy compounds proposed by us (such as resonance energy, strain energy, large π-π separation energy, molecular polarizability, etc.), how these molecular deformability indicators affect the energy and stability of explosive molecules is a question that needs to be answered. In this paper, the large π-π separation energies of large π-π interconnected guanidine derivatives are calculated by the density functional method and the design of isodesmic reactions. The influence of molecular deformability on trigger bonds is revealed, and the understanding of the nature of trigger bonds is improved.
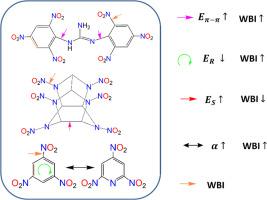
大π-π互联胍基高能化合物及其触发键
TNB、三唑、四唑、呋喃嘧啶、胍等是构建高能化合物的基本构件。不同结构和性质的化合物可以通过不同的方式组合得到(通过原子或基团桥接、尖峰、熔合等)。如何测量它们之间连接的有效性是我们在设计高能化合物时必须考虑的问题。胍是y型芳香化合物,它与其他单个或几个芳香环连接形成大的π-π互连化合物。大π-π分离能可以测量大π-π互连结构由于电子离域而产生的额外稳定能,是表征化合物芳香延伸或芳香性的新指标。也是我们提出的高能化合物分子可变形性的主要指标(如共振能、应变能、大π-π分离能、分子极化率等),这些分子可变形性指标如何影响炸药分子的能量和稳定性是一个需要解答的问题。本文采用密度泛函方法和等径反应设计计算了大π-π互连胍衍生物的大π-π分离能。揭示了分子可变形性对触发键的影响,提高了对触发键性质的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


