A modular and universal platform for neutralizing-antibody profiling by quantifying antigen-receptor interactions via qPCR
IF 2.5
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
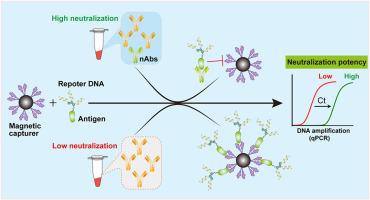
Rapid and accurate detection of neutralizing antibody (nAb) is essential for assessing vaccine efficacy and immune protection against infectious diseases. In the present study, we developed a neutralization-antibody detection qPCR platform (NAD-qPCR) that quantifies nAb potency by integrating antigen-receptor binding specificity with qPCR sensitivity. Using the SARS-CoV-2 nAb as a proof-of-concept target, this system comprised a hybrid probe with the viral RBD as an antigen module covalently conjugated to a reporter DNA, and magnetic beads functionalized with the ACE2 mimic mini-protein as a receptor module, thereby recapitulating the natural antigen-receptor interactions. With this design, the neutralizing capability of nAb against the antigen was transformed into a competitive reduction of the probe binding and DNA amplification signal by qPCR. The NAD-qPCR demonstrated robust performance in quantifying commercial SARS-CoV-2 nAb with a LOD of 4 ng/μL. Furthermore, it was demonstrated to be capable of effectively profiling a large number of vaccinated donor serum with broad neutralization activity. Notably, the hybrid probe and the capture beads were designed modularly, which allows for straightforward adaptation to other pathogens by replacing the module component. This platform offers a rapid, high-throughput, and universally applicable approach for nAb detection in vaccine development and immune monitoring.
通过qPCR定量抗原-受体相互作用的中和抗体分析的模块化和通用平台
快速、准确地检测中和抗体(nAb)对于评估疫苗效力和对传染病的免疫保护至关重要。在本研究中,我们开发了一种中和抗体检测qPCR平台(NAD-qPCR),通过整合抗原受体结合特异性和qPCR敏感性来定量nAb的效力。该系统以SARS-CoV-2 nAb作为概念验证靶点,包括以病毒RBD作为抗原模块共价偶联到报告DNA的杂交探针,以及与ACE2模拟迷你蛋白功能化的磁珠作为受体模块,从而概括了天然抗原-受体相互作用。通过这种设计,将nAb对抗原的中和能力转化为通过qPCR竞争性地减少探针结合和DNA扩增信号。NAD-qPCR在商业SARS-CoV-2 nAb定量中表现出稳健的性能,LOD为4 ng/μL。此外,它被证明能够有效地分析大量具有广泛中和活性的接种供体血清。值得注意的是,杂交探针和捕获珠是模块化设计的,通过替换模块组件,可以直接适应其他病原体。该平台为疫苗开发和免疫监测中的nAb检测提供了一种快速、高通量和普遍适用的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


