CD4 T cell therapy counteracts inflammaging and senescence by preserving gut barrier integrity
IF 16.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
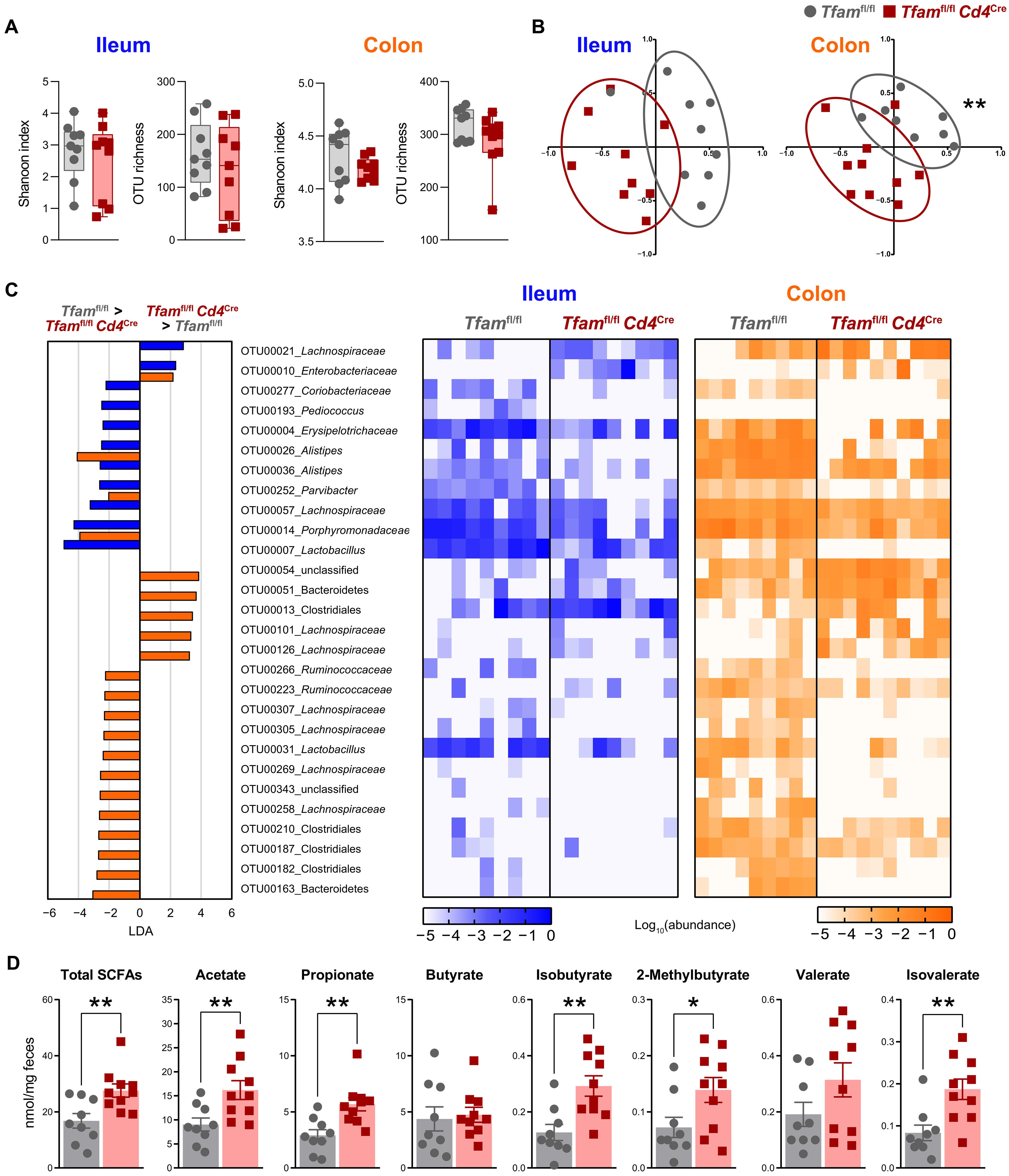
Healthy aging relies on a symbiotic host-microbiota relationship. The age-associated decline of the immune system can pose a threat to this delicate equilibrium. In this work, we investigated how the functional deterioration of T cells can affect host-microbiota symbiosis and gut barrier integrity and the implications of this deterioration for inflammaging, senescence, and health decline. Using the Tfamfl/flCd4Cre mouse model, we found that T cell failure compromised gut immunity leading to a decrease in T follicular cells and regulatory T cells (Treg cells) and an accumulation of highly proinflammatory and cytotoxic T cells. These alterations were associated with intestinal barrier disruption and gut dysbiosis. Microbiota depletion or adoptive transfer of total CD4 T cells or a Treg cell–enriched pool prevented gut barrier dysfunction and mitigated premature inflammaging and senescence, ultimately enhancing the health span in this mouse model. Thus, a competent CD4 T cell compartment is critical to ensure healthier aging by promoting host-microbiota mutualism and gut barrier integrity.
CD4 T细胞疗法通过保持肠道屏障的完整性来抵消炎症和衰老
健康的衰老依赖于宿主-微生物群的共生关系。随着年龄的增长,免疫系统的衰退会对这种微妙的平衡造成威胁。在这项工作中,我们研究了T细胞功能恶化如何影响宿主-微生物群共生和肠道屏障完整性,以及这种恶化对炎症、衰老和健康衰退的影响。使用Tfam fl/fl Cd4 Cre小鼠模型,我们发现T细胞衰竭损害肠道免疫,导致T滤泡细胞和调节性T细胞(T reg细胞)减少,以及高促炎和细胞毒性T细胞的积累。这些改变与肠屏障破坏和肠道生态失调有关。在该小鼠模型中,微生物群消耗或总CD4 T细胞或T细胞富集池的过性转移可防止肠道屏障功能障碍,减轻过早炎症和衰老,最终提高健康寿命。因此,一个合格的CD4 T细胞区室通过促进宿主-微生物群的相互作用和肠道屏障的完整性来确保健康的衰老至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


