Sympathetic innervation maintains the murine quiescent skeletal muscle stem cell pool via perivascular-derived Angpt1
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Muscle stem cells rely on their niche for maintenance, yet how β-adrenergic innervation regulates these cells remains elusive. Here, we show that sympathetic fibers in skeletal muscle innervate the vascular stem cell niche, specifically targeting β-adrenergic receptors on perivascular cells. We observe that sympathetic denervation leads to vascular remodeling and, concomitantly, reduces the muscle stem cell pool, resulting in tissue repair defects. Mechanistically, we demonstrate that sympathetic denervation reduces perivascular-derived angiopoietin-1, a crucial factor in maintaining the quiescent state of post-natal muscle stem cells. Using pharmacologic and genetic tools, we identify that sympathetic signaling drives angiopoietin-1 production from murine perivascular cells through the stimulation of their β-adrenergic receptors, thereby preserving the quiescent stem cell pool. Collectively, our data identify the molecular and cellular axis coupling skeletal muscle tissue homeostasis and regeneration to sympathetic innervation and β-adrenergic signaling, which are thus key signaling pathways that contribute to satellite cell quiescence.
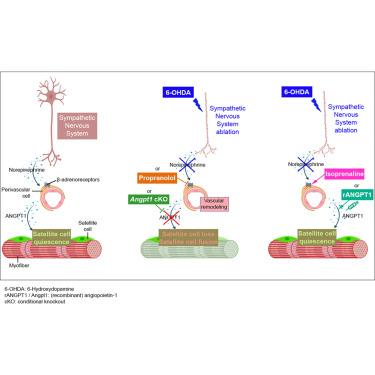
交感神经支配通过血管周围源性Angpt1维持小鼠静止骨骼肌干细胞池
肌肉干细胞依赖于它们的生态位来维持,然而β-肾上腺素能神经支配如何调节这些细胞仍然是一个谜。在这里,我们发现骨骼肌中的交感神经支配血管干细胞生态位,特别针对血管周围细胞上的β-肾上腺素能受体。我们观察到,交感神经去支配导致血管重塑,同时减少肌肉干细胞库,导致组织修复缺陷。在机制上,我们证明了交感神经去支配减少了血管周围来源的血管生成素-1,这是维持出生后肌肉干细胞静止状态的关键因素。使用药理学和遗传学工具,我们发现交感信号通过刺激小鼠血管周围细胞的β-肾上腺素能受体来驱动血管生成素-1的产生,从而保持静止的干细胞池。总的来说,我们的数据确定了分子和细胞轴将骨骼肌组织稳态和再生耦合到交感神经支配和β-肾上腺素能信号传导,因此这是促进卫星细胞静止的关键信号传导途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


