Design of soluble Notch agonists that drive T cell development and boost immunity
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The rational design of receptor agonists to control cell signaling is an emerging strategy for developing disease therapeutics. Creating a soluble cytokine-like agonist for the Notch receptor, which regulates cell fate in embryonic and adult development, is challenging, as receptor activation requires a mechanical force that is usually mediated by cell-associated transmembrane ligands. Here, we exploit computationally designed protein complexes with precise valencies and geometries to generate soluble cytokine-like Notch agonists. These molecules promote cell-cell bridging, cluster Notch receptors at cell synapses, and activate receptor signaling. We show that these agonists drive T cell differentiation from cord blood progenitors and human induced pluripotent stem cells (iPSCs) and in bioreactor production of T cells in liquid suspension. When delivered intravenously in mice, they stimulate cytokine production, expansion of antigen-specific CD4+ T cells, and antibody class switching. These de-novo-designed ligands can be broadly applied to optimize in vitro cell differentiation and advance immunotherapy development.
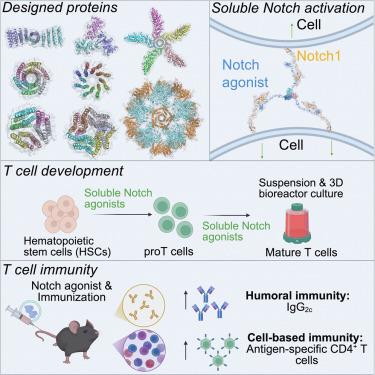
可溶性Notch激动剂的设计,驱动T细胞发育和增强免疫力
合理设计受体激动剂来控制细胞信号传导是发展疾病治疗的新兴策略。Notch受体在胚胎和成人发育过程中调节细胞命运,为Notch受体创造一种可溶性细胞因子样激动剂是具有挑战性的,因为受体激活需要机械力,而机械力通常由细胞相关的跨膜配体介导。在这里,我们利用计算设计的具有精确价和几何形状的蛋白质复合物来生成可溶性细胞因子样Notch激动剂。这些分子促进细胞间桥接,在细胞突触聚集Notch受体,并激活受体信号。我们发现这些激动剂驱动T细胞从脐带血祖细胞和人诱导多能干细胞(iPSCs)分化,并在液体悬浮液中生物反应器生产T细胞。当给小鼠静脉注射时,它们刺激细胞因子的产生,抗原特异性CD4+ T细胞的扩增和抗体类别转换。这些新设计的配体可以广泛应用于优化体外细胞分化和推进免疫治疗的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


