Plasma membrane CYBDOM proteins catalyse apoplastic AsA regeneration and interact with RbohD to activate autophagy and drought tolerance in plants
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
Autophagy and apoplastic ascorbic acid (AsA) play important roles in plant drought tolerance. However, the trans-plasma membrane transport of AsA and its association with autophagy during drought stress are unclear. Here we report an AsA-induced autophagy pathway in plants, wherein plasma-membrane-located cytochrome b561 and DOMON domain (CYBDOM) proteins play positive roles. CYBDOM proteins from the resurrection plant Boea hygrometrica (BhDB) and Arabidopsis (AtDB1) can transport electrons across the plasma membrane using intracellular AsA as an electron donor and apoplastic monodehydroascorbic acid or Fe3+ as electron acceptors in Xenopus laevis oocytes. Increased apoplastic AsA, autophagy and drought tolerance are observed in BhDB- and AtDB1-overexpressing Arabidopsis compared with the wild type. CYBDOM proteins interact with respiratory burst oxidase homologue D (RbohD), which serves as the adaptor to bind autophagy related gene 8 protein (ATG8) and cargo protein for autophagic degradation. AsA increases AtDB1 protein level and its interaction with RbohD. Together, the AsA-activated CYBDOM–RbohD synergy to induce autophagy suggests a novel mechanism in plant drought and desiccation tolerance. This study reveals that CYBDOM proteins promote drought tolerance by regenerating apoplastic ascorbic acid (AsA) and activating autophagy through interaction with RbohD, and link AsA redox, ROS and selective autophagy in plant stress responses
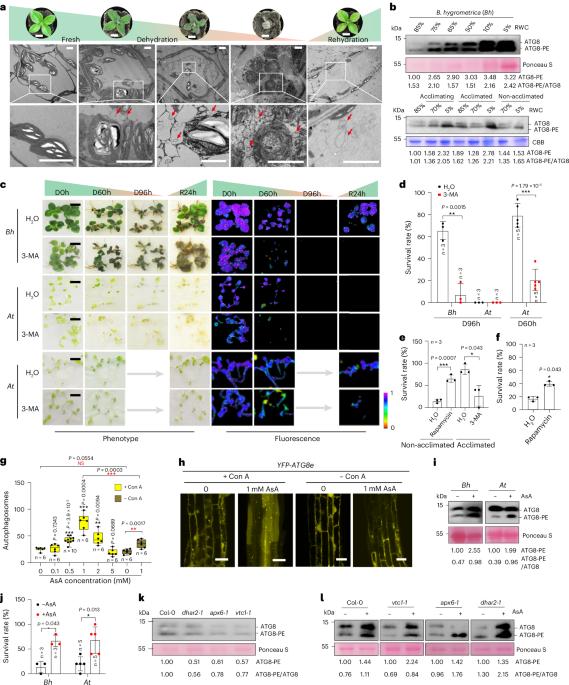

质膜CYBDOM蛋白催化外胞体AsA再生,并与RbohD相互作用,激活植物自噬和抗旱性
自噬和抗坏血酸(AsA)在植物抗旱性中起着重要作用。然而,干旱胁迫下AsA的跨质膜转运及其与自噬的关系尚不清楚。在这里,我们报道了asa诱导的植物自噬途径,其中质膜定位的细胞色素b561和DOMON结构域(CYBDOM)蛋白发挥了积极作用。在非洲蟾卵母细胞中,来自复活植物Boea hygrometrica (BhDB)和拟南芥(AtDB1)的CYBDOM蛋白可以通过胞内AsA作为电子供体和外胞单脱氢抗坏血酸或Fe3+作为电子受体在质膜上传递电子。与野生型相比,过表达BhDB-和atdb1的拟南芥的AsA、自噬和耐旱性均有所增加。CYBDOM蛋白与呼吸爆发氧化酶同源物D (RbohD)相互作用,RbohD作为接头结合自噬相关基因8蛋白(ATG8)和自噬降解货物蛋白。AsA增加AtDB1蛋白水平及其与RbohD的相互作用。综上所述,asa激活的CYBDOM-RbohD协同诱导自噬提示了植物干旱和干燥耐受的新机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


