Bim gene regulation in hypoxic stress response of blunt snout bream (Megalobrama amblycephala): Mechanisms of apoptosis, oxidative stress, and transcriptional control by c-Ets-2
IF 2.2
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology
Pub Date : 2025-07-28
DOI:10.1016/j.cbpa.2025.111913
引用次数: 0
Abstract
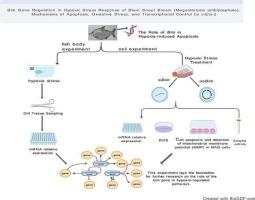
Despite its significant economic value, the blunt snout bream (Megalobrama amblycephala) is extremely sensitive to hypoxia. Derived from blunt snout bream gill filament cell lines, Megalobrama amblycephala gill (MAG) cells are closely linked to hypoxia, making them suitable for identifying related genes' hypoxia tolerance. This study explores the roles of the Bcl-2 interacting mediator of cell death gene (Bim) in the gill tissues and MAG cells under hypoxic conditions. Bim expression increased significantly (P < 0.05) after 24 h of hypoxia but decreased following reoxygenation. Overexpression of Bim significantly (P < 0.05) upregulated the expression of pro-apoptotic genes Bax and Caspase 3, while downregulating the expression of the anti-apoptotic gene Bcl-2. Conversely, Bim interference exhibited an opposite trend. Hypoxia led to increased apoptosis and decreased mitochondrial membrane potential (MMP) in MAG cells, which was exacerbated by overexpression of Bim. In contrast, Bim interference attenuated apoptosis and prevented the decrease in MMP. ROS levels significantly (P < 0.05) increased under hypoxic conditions, and Bim overexpression further elevated ROS production, whereas Bim interference reduced ROS levels. Antioxidant enzyme (SOD, CAT) activity decreased after hypoxia, which was exacerbated by Bim overexpression, while Bim interference slowed the decline of enzyme activity. The dual-luciferase reporter assay confirmed that the transcription factor c-Ets-2 regulates the expression of Bim by binding to the -GAGGAA site of the Bim promoter. This study highlights Bim's key role in hypoxic stress and offers new insights into the hypoxic adaptation mechanisms of blunt snout bream.
Bim基因在钝口鲷(Megalobrama amblycephala)缺氧应激反应中的调控:凋亡、氧化应激和c-Ets-2转录调控的机制
钝口鲷(Megalobrama amblycepphala)尽管具有重要的经济价值,但对缺氧极为敏感。Megalobrama amblycephala gill (MAG)细胞来源于钝口鲷鳃丝细胞系,与缺氧密切相关,适合用于鉴定缺氧耐受相关基因。本研究探讨缺氧条件下细胞死亡基因(Bim)的Bcl-2相互作用介质在鳃组织和MAG细胞中的作用。Bim表达显著升高(P
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.00
自引率
4.30%
发文量
155
审稿时长
3 months
期刊介绍:
Part A: Molecular & Integrative Physiology of Comparative Biochemistry and Physiology. This journal covers molecular, cellular, integrative, and ecological physiology. Topics include bioenergetics, circulation, development, excretion, ion regulation, endocrinology, neurobiology, nutrition, respiration, and thermal biology. Study on regulatory mechanisms at any level of organization such as signal transduction and cellular interaction and control of behavior are also published.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


