De-risking vaccine development: lessons, challenges, and prospects.
IF 6.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
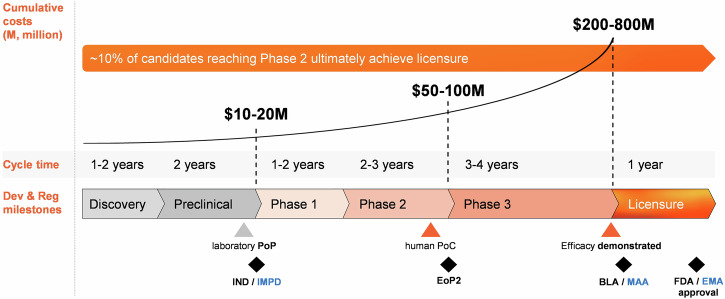
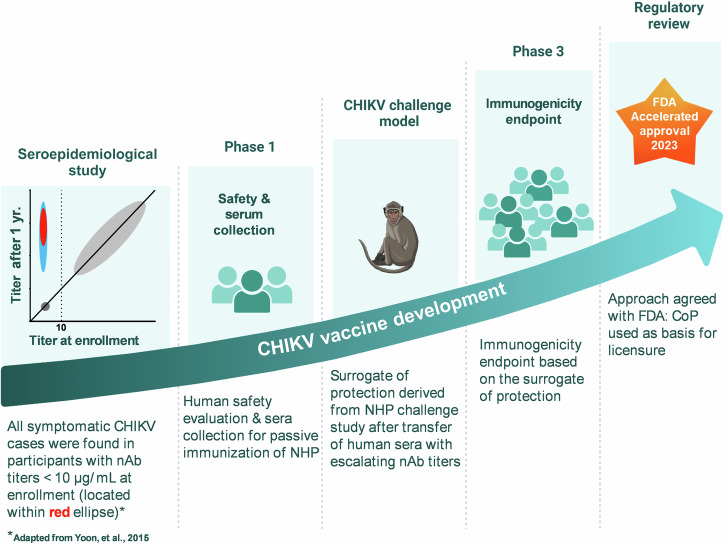
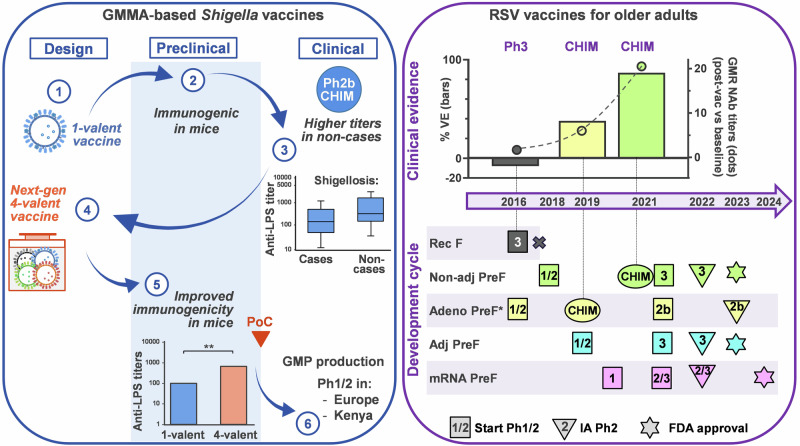
Vaccine development is long and costly. Technical, clinical, regulatory, and manufacturing risks throughout the development cycle hampered manufacturers' efforts towards more challenging and financially less rewarding targets, with profound implications for public health. Early de-risking strategies can help closing the vaccine productivity gap and support sustainable access to vaccines. Illustrated by examples of early and efficient decision-making, the herein proposed strategies can ultimately increase the success rates of vaccine programs.
降低疫苗开发风险:经验教训、挑战和前景。
疫苗开发耗时长,成本高。整个开发周期的技术、临床、监管和生产风险阻碍了制造商实现更具挑战性和财务回报较低的目标的努力,对公共卫生产生了深远影响。早期降低风险战略有助于缩小疫苗生产力差距并支持可持续获得疫苗。通过早期有效决策的例子说明,本文提出的策略最终可以提高疫苗计划的成功率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Vaccines
Immunology and Microbiology-Immunology
CiteScore
11.90
自引率
4.30%
发文量
146
审稿时长
11 weeks
期刊介绍:
Online-only and open access, npj Vaccines is dedicated to highlighting the most important scientific advances in vaccine research and development.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


