Application of cosolvency and cocrystallization approach to enhance acyclovir solubility
Abstract
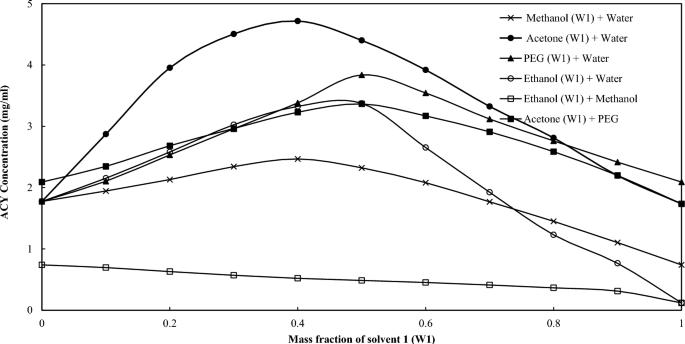
Acyclovir (ACY) is a commonly used antiviral drug with limited aqueous solubility and poor permeability. It is administered via oral and intravenous routes. The aim of this work is to employ cocrystallization and cosolvency method to improve the solubility of orally administered dosage forms, including tablet and oral solution. Cocrystallization is carried out through the solvent evaporation method using different types of coformers including formic acid (FA), tartaric acid (TA), and ascorbic acid (AA). The physical states of the obtained samples were characterized by differential scanning calorimeter (DSC), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR). The solubility of the developed samples were evaluated and compared with those of the parent drug. DSC and XRD findings indicate that ACY-AA and ACY-TA may be classified as cocrystals. Physicochemical characterization of ACY-FA did not demonstrate any change with respect to the parent drug. ACY-AA had the highest increase in drug solubility and created almost 3 folds enhancement. In another approach, cosolvency was employed to study the solubilization enhancement of 6 different binary solvent mixtures at 298.15 K. PEG 400 + water at 0.5:0.5 demonstrated a twofold enhancement in drug solubility. Both cocrystallization and cosolvency method seem effective in enhancing drug solubility and have the potential to be employed for the development of oral drug delivery systems including tablets and oral suspensions. The findings of this work suggest that ACY solubility can be enhanced by proper choice of the coformer and cosolvent. The result of this finding is beneficial for the development of acyclovir dosage forms.
Graphical abstract

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


