Rapid access to arynes from aryl triflates using a non-competitive, commercially available amide base
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Arynes are novel intermediates for organic synthesis and aryl triflates are potentially useful aryne precursors because they are commercially available or easily prepared from widely abundant phenols. However, strong bases, which are also competitive arynophiles, are often used to generate arynes from aryl triflates and this can limit their use in synthesis. We have found that the commercially available lithium bis(trimethylsilyl)amide is uniquely non-competitive among this class of base in generating arynes from aryl triflates. We demonstrate that this method is compatible with 15 aryl triflates and 6 different arynophiles in yields ranging from 54 to 96 % (75 % avg). Moreover, we parameterize several common bases used to generate arynes from aryl triflates on an arynophilicity scale, and we describe the overall robustness of this process relative to other aryne forming reactions.
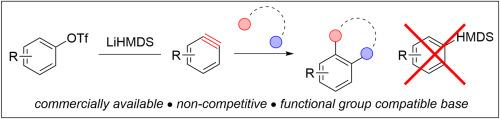
从芳基三氟酸盐中快速获得芳烃,使用非竞争性的,市售的酰胺基
芳香烃是一种新型的有机合成中间体,三氟化芳基酯是一种潜在的有用的芳香烃前体,因为它们可以在市场上买到,或者很容易从广泛丰富的酚中制备出来。然而,强碱(也是竞争性亲芳试剂)通常用于从芳基三氟酸酯生成芳炔,这限制了它们在合成中的使用。我们发现,市售的双(三甲基硅基)锂酰胺在从三氟化芳基生成芳炔方面,在这类碱中具有独特的非竞争性。结果表明,该方法与15种芳基三氟酸酯和6种不同的亲芳香剂兼容,产率在54% ~ 96%(平均75%)之间。此外,我们在亲芳性尺度上参数化了用于从芳基三氟酸酯生成芳烷的几种常见碱,并描述了该过程相对于其他芳烷形成反应的总体稳健性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


