Fe-Cu-loaded γ-Al2O3 as an efficient heterogeneous Fenton catalyst for the advanced treatment of petrochemical wastewater
IF 8.7
Q1 Environmental Science
引用次数: 0
Abstract
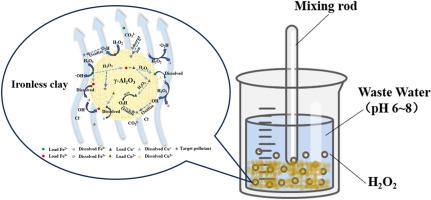
A novel Fe-Cu/γ-Al2O3 heterogeneous Fenton catalyst was developed for the degradation of real petrochemical secondary effluent using H2O2 under near-neutral pH conditions (6–8). This approach addresses key limitations of traditional Fenton systems, including their narrow pH range and generation of iron sludge. No sludge formation was detected, and Fe/Cu leaching remained below 1 mg/L after nine consecutive reuse cycles, confirming the catalyst's stability and reusability. The catalyst was synthesized via impregnation and calcination, with preparation parameters optimized using a one-factor-at-a-time method. Under optimal conditions (Fe: 0.28 mol/L; Cu: 0.20 mol/L; 500 °C; 7 h), 50.15% TOC removal was achieved using 20 g catalyst and 13.73 mmol/L H2O2. Characterization techniques (SEM, BET, XRD, XPS) confirmed the structural features, while EPR and quenching experiments revealed that ·OH and HO2− were the dominant reactive species. The Fe(III)/Fe(II) and Cu(II)/Cu(I) redox pairs on the catalyst surface were identified as active sites for H2O2 activation. This study demonstrates a robust, sludge-free Fenton-like system with practical potential for advanced petrochemical wastewater treatment.
fe - cu负载γ-Al2O3作为石化废水深度处理的高效非均相Fenton催化剂
研究了一种新型Fe-Cu/γ-Al2O3非均相Fenton催化剂,用于在接近中性pH条件下使用H2O2降解石化二次出水(6-8)。这种方法解决了传统Fenton系统的主要局限性,包括其狭窄的pH范围和产生铁污泥。连续9次重复使用后,未发现污泥形成,Fe/Cu浸出率保持在1 mg/L以下,证实了催化剂的稳定性和可重复使用性。采用浸渍法和煅烧法合成催化剂,并采用单因素法优化制备参数。在最佳条件下(Fe: 0.28 mol/L;Cu: 0.20 mol/L;500°C;7 h),催化剂用量为20 g, H2O2用量为13.73 mmol/L, TOC去除率为50.15%。表征技术(SEM, BET, XRD, XPS)证实了结构特征,而EPR和淬火实验表明·OH和HO2−是主要的反应物质。催化剂表面的Fe(III)/Fe(II)和Cu(II)/Cu(I)氧化还原对被确定为H2O2活化的活性位点。该研究展示了一个强大的、无污泥的类芬顿系统,具有先进的石化废水处理的实际潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Cycle
Engineering-Engineering (miscellaneous)
CiteScore
9.20
自引率
0.00%
发文量
20
审稿时长
45 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


