Analysis of the distribution of HEV by immunohistochemistry using a mouse oral squamous cell carcinoma model
IF 2.3
Q1 DENTISTRY, ORAL SURGERY & MEDICINE
引用次数: 0
Abstract
Objective
The density of tumor-associated high endothelial venule (TA-HEV) correlates with a favorable prognosis in various tumors, including oral squamous cell carcinoma (OSCC). Despite the association of TA-HEVs with a better prognosis, their changes during tumor progression remain unclear. Therefore, this study aimed to examine these alterations in a mouse OSCC model.
Methods
Mice were treated with water containing 4NQO to establish a mouse OSCC model. Immunohistochemical analyses were performed using CD31 and MECA 79 antibodies to evaluate TA-HEV alterations during tumor progression.
Results
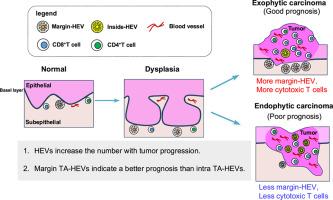
Tongue samples were histologically classified into untreated, normal, dysplasia, and carcinoma groups. During the dysplastic stage, the number of blood vessels increased, and pericyte coverage was reduced as the carcinoma developed. Moreover, HEVs were more abundant in the dysplasia and carcinoma groups, with an increased number of HEVs infiltrated with CD8+ T cells as the lesion progressed. The location and morphology of the HEVs changed during the transition from normal tissue to carcinoma, with the distribution of the TA-HEVs associated with the tumor growth patterns. Specifically, TA-HEVs were frequently found within the tumor in endophytic carcinomas, and at the tumor margins in exophytic carcinomas. Margin TA-HEVs correlated with a higher number of T cells than HEVs within the tumors.
Conclusion
OSCC-associated HEVs increase in density with tumor progression, and margin TA-HEVs suggests a better anti-tumor prognosis compared with intra-TA-HEVs.
利用小鼠口腔鳞状细胞癌模型免疫组织化学方法分析HEV的分布
目的肿瘤相关高内皮小静脉(TA-HEV)的密度与包括口腔鳞状细胞癌(OSCC)在内的多种肿瘤的良好预后相关。尽管ta - hev与较好的预后相关,但其在肿瘤进展过程中的变化尚不清楚。因此,本研究旨在检测小鼠OSCC模型中的这些改变。方法采用含4NQO水处理小鼠OSCC模型。使用CD31和MECA 79抗体进行免疫组织化学分析,以评估TA-HEV在肿瘤进展过程中的改变。结果舌样在组织学上分为未治疗组、正常组、不典型增生组和癌组。在发育不良阶段,随着癌的发展,血管数量增加,周细胞覆盖减少。此外,hev在非典型增生组和癌组中更丰富,随着病变的进展,hev浸润CD8+ T细胞的数量增加。在正常组织向癌组织转变的过程中,hev的位置和形态发生了变化,ta - hev的分布与肿瘤的生长模式有关。具体来说,ta - hev在内生癌的肿瘤内和外生性癌的肿瘤边缘经常被发现。边缘ta - hev与肿瘤内T细胞数量的相关性高于hev。结论随着肿瘤进展,oscc相关hev的密度增加,边缘ta - hev比ta - hev具有更好的抗肿瘤预后。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Oral Biosciences
DENTISTRY, ORAL SURGERY & MEDICINE-
CiteScore
4.40
自引率
12.50%
发文量
57
审稿时长
37 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


