Kinetic Resolution of Secondary Alcohols Using Connon Type Catalysts
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Chiral pyridine derivatives following the general design principle proposed by Connon et al. are employed in the Lewis base mediated kinetic resolution of secondary alcohols. Variations in the size of substrates and catalyst substituents are found to impact reaction selectivity in a nonsystematic manner, while best selectivities are found for catalyst variants carrying electron‐acceptor‐substituted side chains. Solvent effects are found to be small for the most active and most selective catalysts studied, but more significant for less selective catalysts. The quantitative assessment of reaction kinetics points to a rather limited contribution of background acylation reactivity, but to a very significant temperature effect on reaction outcome.
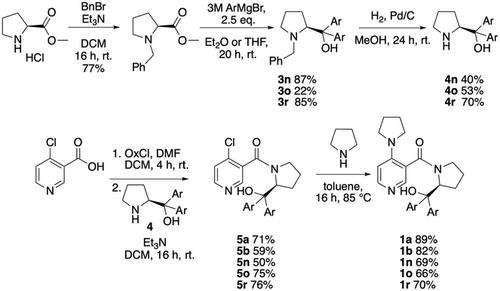
用Connon型催化剂动力学拆分仲醇
手性吡啶衍生物遵循Connon等人提出的一般设计原则,用于Lewis碱介导的仲醇动力学拆分。研究发现,底物和催化剂取代基的大小变化会以非系统的方式影响反应选择性,而携带电子受体取代侧链的催化剂变体具有最佳选择性。研究发现,对于大多数活性催化剂和选择性催化剂,溶剂效应很小,但对于选择性较低的催化剂,溶剂效应更为显著。反应动力学的定量评估表明,背景酰化反应活性的贡献相当有限,但温度对反应结果的影响非常显著。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


