Profiles of extracellular vesicle shutter proteins from Chlamys farreri stimulated by lipopolysaccharide
IF 2.4
3区 农林科学
Q1 FISHERIES
引用次数: 0
Abstract
Extracellular vesicles (EVs) in aquatic animals can resist pathogen infection by delivering bioactive substances between cells. Chlamys farreri, as a commercial aquaculture species, was used as the research object in this study to explore the possible innate immune regulation mediated by EVs. In this study, data-independent acquisition (DIA) proteomics analysis was performed on EVs in the serum of C. farreri after lipopolysaccharide (LPS) stimulation. A total of 124 upregulated and 205 downregulated differentially expressed proteins (DEPs) were identified through comparative analysis. Related DEPs that may be involved in immune regulation in EVs were further screened, such as glucose-regulated protein 94 (HSP90B1), lysosome-associated membrane glycoprotein 1 (LAMP1), calreticulin (CALR), ras-related protein Rab-11 A (RAB11A), serine/arginine-rich splicing factor 1 (SRSF1), tumor susceptibility gene 101 protein (TSG101), and vesicle-associated membrane protein 3 (VAMP3). Enrichment analysis demonstrated that these DEPs were identified to be involved in the response to stimulus function, immune system process, and classical immune-related signaling pathways, such as the Toll-like receptor signaling pathway and RIG-I-like receptor signaling pathway. Further, PPI analysis was performed to predict the interactions between immune-related DEPs, which were mainly divided into proteins directly involved in immune regulation (such as HSP90B1, LAMP1, CALR, and RAB11A) and proteins indirectly involved in immune regulation by vesicle transport (such as TSG101, VAMP3, SNAP25, RAB14, and RAB2). These findings reveal that EVs play a central role in mediating innate immune regulation in C. farreri via cell communication under LPS stimulation, and are expected to provide important insights into disease-resistant breeding and aquaculture practices in shellfish.
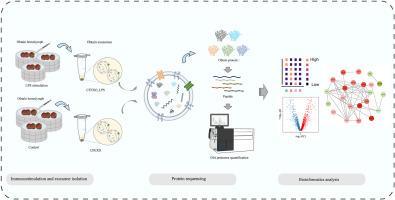
脂多糖刺激下栉孔扇贝胞外囊泡关闭蛋白的谱图
水生动物的细胞外囊泡(EVs)通过在细胞间传递生物活性物质来抵抗病原体感染。本研究以商业养殖物种栉孔栉孔虾(Chlamys farreri)为研究对象,探讨ev介导的先天免疫调节机制。本研究采用数据独立采集(DIA)蛋白质组学方法对脂多糖(LPS)刺激后法氏梭菌血清中的ev进行分析。通过比较分析,共鉴定出124个上调的差异表达蛋白和205个下调的差异表达蛋白(DEPs)。进一步筛选可能参与EVs免疫调节的相关DEPs,如葡萄糖调节蛋白94 (HSP90B1)、溶酶体相关膜糖蛋白1 (LAMP1)、钙网蛋白(CALR)、ras相关蛋白rab - 11a (RAB11A)、丝氨酸/精氨酸丰富剪接因子1 (SRSF1)、肿瘤易感基因101蛋白(TSG101)、囊泡相关膜蛋白3 (VAMP3)等。富集分析表明,这些dep参与刺激功能应答、免疫系统过程和经典免疫相关信号通路,如toll样受体信号通路和rig - i样受体信号通路。进一步,通过PPI分析预测免疫相关DEPs之间的相互作用,DEPs主要分为直接参与免疫调节的蛋白(如HSP90B1、LAMP1、CALR和RAB11A)和通过囊泡运输间接参与免疫调节的蛋白(如TSG101、VAMP3、SNAP25、RAB14和RAB2)。这些发现表明,在LPS刺激下,EVs通过细胞通讯介导法氏梭菌的先天免疫调节,并有望为贝类的抗病育种和养殖实践提供重要见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.20
自引率
6.90%
发文量
206
审稿时长
49 days
期刊介绍:
Developmental and Comparative Immunology (DCI) is an international journal that publishes articles describing original research in all areas of immunology, including comparative aspects of immunity and the evolution and development of the immune system. Manuscripts describing studies of immune systems in both vertebrates and invertebrates are welcome. All levels of immunological investigations are appropriate: organismal, cellular, biochemical and molecular genetics, extending to such fields as aging of the immune system, interaction between the immune and neuroendocrine system and intestinal immunity.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


