First-principles study on the role of Ti, V, and Sc catalysts in enhancing the catalytic effects of boron oxide monolayer for efficient Lithium-selenium batteries
IF 6.2
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
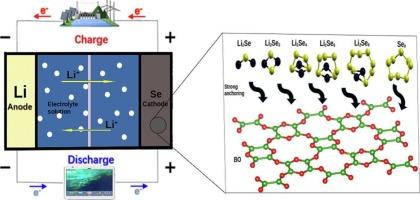
Ongoing research on lithium‑selenium batteries (LiSeB) aims to overcome setbacks caused by shuttle effects by exploring various cathode additive materials, with a particular focus on 2D materials. These materials are gaining popularity because of their unique properties, such as large surface areas, ballistic electronic transport, mechanical strength, and anisotropy, making them promising candidates for cathode additives in LiSeB. In this study, density functional theory (DFT) was used to investigate the interaction of lithium polyselenides (specifically Li2Sex where x = 1, 2, 4, 6, and 8, as well as Se8) on recently synthesized boron monoxide monolayer (BO). We investigated the influence of Li2Sex and Se8 on BO, focusing on the adsorption energy, the charge density distribution, Gibbs free energy changes, and the metallic characteristics for efficient LiSeB. The results showed that the adsorption energies of these Li2Sex and Se8 on pristine BO are relatively weak, ranging from −0.25 to −1.43 eV. In contrast, doping BO with scandium (Sc) significantly increased the adsorption energies, ranging from −2.65 to −3.74 eV, indicating a notable enhancement compared to other single-atom catalysts (SACs). The strong adsorption energy of Sc-doped BO suggested an improved ability to prevent the dissociation of Li2Sex and Se8 in the electrolyte, which is critical to address the notorious shuttle effects. Charge density distribution analyses further supported the presence of electronic interactions between the substrate and the adsorbed Li2Sex and Se8 via Sc catalysts, as evidenced by charge transfer from the adsorbate to the substrate. Furthermore, the investigation of Gibbs free energies revealed low charge, discharge, and overpotential values (0.1 V for pristine BO and 1.53 V for Sc-doped BO). The Sc-doped BO structure exhibited significantly enhanced metallic characteristics after adsorption of Li2Se and Li2Se4. Furthermore, the low diffusion (1.56 eV) and dissociation (1.72 eV) energy barriers for stable Li2Se on Sc-doped BO suggested the material's potential to improve electrochemical processes and enable higher charging rates in LiSeB. Ultimately, while pristine BO alone may not effectively address the challenges associated with LiSeB, doping it with Sc substantially enhances its properties as a cathode additive.
Ti、V、Sc催化剂增强氧化硼单层对高效锂硒电池催化效果的第一性原理研究
锂硒电池(LiSeB)正在进行的研究旨在通过探索各种阴极添加剂材料,特别是2D材料,来克服由穿梭效应引起的挫折。这些材料由于其独特的性能(如大表面积、弹道电子输运、机械强度和各向异性)而越来越受欢迎,使其成为LiSeB中阴极添加剂的有希望的候选者。在这项研究中,密度泛函理论(DFT)被用于研究锂多硒化物(特别是Li2Sex,其中x = 1,2,4,6和8,以及Se8)在最近合成的一氧化硼单层(BO)上的相互作用。研究了Li2Sex和Se8对BO的影响,重点研究了高效LiSeB的吸附能、电荷密度分布、吉布斯自由能变化和金属特性。结果表明,Li2Sex和Se8在原始BO上的吸附能较弱,在−0.25 ~−1.43 eV之间。相比之下,BO与钪(Sc)的掺杂显著提高了吸附能,吸附能在−2.65 ~−3.74 eV之间,与其他单原子催化剂(SACs)相比有显著的增强。sc掺杂的BO具有很强的吸附能,这表明它可以提高电解质中Li2Sex和Se8的解离能力,这对解决臭名昭著的穿梭效应至关重要。电荷密度分布分析进一步支持了基体与Sc催化剂吸附的Li2Sex和Se8之间存在电子相互作用,从吸附物到基体的电荷转移证明了这一点。此外,对吉布斯自由能的研究表明,其电荷、放电和过电位值较低(原始BO为0.1 V,掺杂sc的BO为1.53 V)。在吸附Li2Se和Li2Se4后,掺杂sc的BO结构表现出明显增强的金属特性。此外,稳定Li2Se在sc掺杂BO上的低扩散(1.56 eV)和解离(1.72 eV)能垒表明该材料具有改善电化学过程和提高LiSeB充电速率的潜力。最终,虽然单纯纯净的BO可能无法有效解决与LiSeB相关的挑战,但掺杂Sc可以显著提高其作为阴极添加剂的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

FlatChem
Multiple-
CiteScore
8.40
自引率
6.50%
发文量
104
审稿时长
26 days
期刊介绍:
FlatChem - Chemistry of Flat Materials, a new voice in the community, publishes original and significant, cutting-edge research related to the chemistry of graphene and related 2D & layered materials. The overall aim of the journal is to combine the chemistry and applications of these materials, where the submission of communications, full papers, and concepts should contain chemistry in a materials context, which can be both experimental and/or theoretical. In addition to original research articles, FlatChem also offers reviews, minireviews, highlights and perspectives on the future of this research area with the scientific leaders in fields related to Flat Materials. Topics of interest include, but are not limited to, the following: -Design, synthesis, applications and investigation of graphene, graphene related materials and other 2D & layered materials (for example Silicene, Germanene, Phosphorene, MXenes, Boron nitride, Transition metal dichalcogenides) -Characterization of these materials using all forms of spectroscopy and microscopy techniques -Chemical modification or functionalization and dispersion of these materials, as well as interactions with other materials -Exploring the surface chemistry of these materials for applications in: Sensors or detectors in electrochemical/Lab on a Chip devices, Composite materials, Membranes, Environment technology, Catalysis for energy storage and conversion (for example fuel cells, supercapacitors, batteries, hydrogen storage), Biomedical technology (drug delivery, biosensing, bioimaging)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


