Microscopic insights into the initial oxidation process of single crystalline platinum group metal surfaces: From subsurface oxygen, a ghost species, towards surface oxide
IF 8.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this review, the initial oxidation process of low-index surfaces of single-crystalline platinum group metals (PGMs: Ru, Rh, Pd, Ir, and Pt) is discussed in detail at the atomic level, involving several types of oxygen species: chemisorbed O, subsurface O, dissolved O, oxidic O. The oxidation of PGMs begins only when the surface of the PGM is saturated with chemisorbed O. Oxygen penetration into the metal is a critical next step in surface oxide formation, which can occur either through the step edge or directly through the terrace, depending on the oxidants chosen (O2, NO2, atomic O, and ozone O3). However, subsurface oxygen (oxygen directly below the top metal layer) does not form a separate phase in PGM. Instead, a surface oxide consisting of a single O-Me-O trilayer nucleates and grows (heterogeneous growth mode). The oxidation process is a nonlinear process with self-acceleration and passivation behavior, where many processes occur in parallel and in sequence, so that patterning can occur on different length scales. For this reason, oxidation studies must be performed at both the atomic and mesoscale using powerful combinations of surface science techniques such as scanning tunneling microscopy (STM) and low-energy electron microscopy (LEEM).
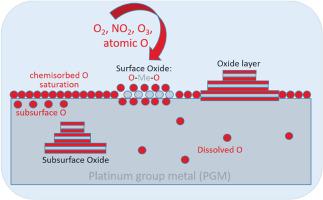
微观观察单晶铂族金属表面的初始氧化过程:从地下氧,一个幽灵物种,到表面氧化物
本文在原子水平上详细讨论了单晶铂族金属(铂族金属:Ru、Rh、Pd、Ir和Pt)低指数表面的初始氧化过程,涉及几种类型的氧:化学吸附O、亚表面O、溶解O、氧化O.只有当化学吸附O使PGM表面饱和时,PGM的氧化才开始。氧渗透到金属中是表面氧化物形成的关键步骤,它可以通过台阶边缘或直接通过台阶发生,这取决于所选择的氧化剂(O2、NO2、原子O和臭氧O3)。然而,在PGM中,亚表面氧(直接在上层金属层下方的氧)不会形成单独的相。相反,由单一O-Me-O三层组成的表面氧化物会成核并生长(非均相生长模式)。氧化过程是一个具有自加速和钝化行为的非线性过程,其中许多过程并行和顺序发生,因此图案可以在不同的长度尺度上发生。由于这个原因,氧化研究必须在原子和中尺度上进行,使用表面科学技术的强大组合,如扫描隧道显微镜(STM)和低能电子显微镜(LEEM)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Surface Science Reports
化学-物理:凝聚态物理
CiteScore
15.90
自引率
2.00%
发文量
9
审稿时长
178 days
期刊介绍:
Surface Science Reports is a journal that specializes in invited review papers on experimental and theoretical studies in the physics, chemistry, and pioneering applications of surfaces, interfaces, and nanostructures. The topics covered in the journal aim to contribute to a better understanding of the fundamental phenomena that occur on surfaces and interfaces, as well as the application of this knowledge to the development of materials, processes, and devices. In this journal, the term "surfaces" encompasses all interfaces between solids, liquids, polymers, biomaterials, nanostructures, soft matter, gases, and vacuum. Additionally, the journal includes reviews of experimental techniques and methods used to characterize surfaces and surface processes, such as those based on the interactions of photons, electrons, and ions with surfaces.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


