Preparation and osteogenic performance study of troxerutin-loaded carboxymethyl cellulose/Si-calcium phosphate cement composite bone cement
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-07-28
DOI:10.1016/j.bioadv.2025.214434
引用次数: 0
Abstract
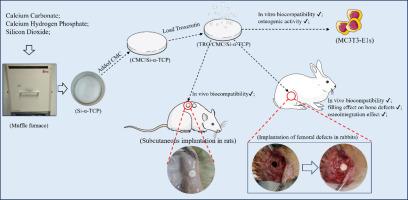
Bone defects from trauma, infection, and tumour resection are a growing clinical challenge due to global population aging. Current treatments like autologous and allogeneic bone grafting have limitations. This study focused on optimizing ion-doped α-tricalcium phosphate (α-TCP) preparation and developing calcium phosphate cement (CPC) with superior physicochemical and biological properties. Calcium pyrophosphate (CPP) and calcium carbonate (CaCO₃) showed the highest conversion efficiency during synthesis. Adding SiO₂ to this combination generated high-purity silicon-doped α-TCP (Si-α-TCP) powder at 1200 °C. To enhance CPC's performance, different amounts of sodium carboxymethyl cellulose (CMC) were added to the setting liquid. The CMC/Si-CPC with 1 %wt CMC demonstrated the best physicochemical properties, with improved setting time, compressive strength, injectability, and anti-dispersion. In drug-loading experiments, CMC promoted the release of Troxerutin (TRO), showing burst release within 6 h followed by sustained release. In vitro experiments with MC3T3-E1 cells confirmed good biocompatibility and osteogenic activity, further enhanced by Si-ion doping and 0.5 mg/mL TRO in the setting liquid. In vivo experiments, including rat subcutaneous and rabbit femoral defect implantation, confirmed effective osteoconductivity and osseointegration without inflammation or necrosis. In conclusion, this study successfully prepared high-purity Si-α-TCP powder by optimizing raw material combinations and Si-ion doping. CMC improved CPC's physicochemical properties, while Si-ion doping and TRO loading enhanced its biocompatibility and osteogenic activity. TRO/CMC/Si-CPC is promising for bone defect treatment and offers a new concept for bone tissue engineering materials.
羧甲基纤维素/硅钙磷酸酯复合骨水泥的制备及成骨性能研究
由于全球人口老龄化,创伤、感染和肿瘤切除引起的骨缺损是一个日益增长的临床挑战。目前的治疗方法如自体和异体骨移植有局限性。本研究旨在优化离子掺杂α-磷酸三钙(α-TCP)的制备工艺,开发具有优异理化和生物性能的磷酸钙水泥(CPC)。焦磷酸钙(CPP)和碳酸钙(CaCO₃)在合成过程中转化效率最高。在此混合物中加入SiO₂,在1200℃下得到高纯掺硅α-TCP (Si-α-TCP)粉末。在定型液中加入不同量的羧甲基纤维素钠(CMC),以提高CPC的性能。含有1% CMC的CMC/Si-CPC表现出最佳的物理化学性能,凝结时间、抗压强度、注射性和抗分散性都有所改善。在载药实验中,CMC促进了Troxerutin (TRO)的释放,在6 h内呈爆发性释放,随后呈缓释。体外实验证实MC3T3-E1细胞具有良好的生物相容性和成骨活性,并通过硅离子掺杂和0.5 mg/mL TRO的培养液进一步增强。体内实验,包括大鼠皮下和兔股骨缺损植入,证实有效的骨传导和骨整合,无炎症或坏死。综上所述,本研究通过优化原料组合和硅离子掺杂,成功制备了高纯度Si-α-TCP粉体。CMC改善了CPC的理化性能,而Si-ion掺杂和TRO负载增强了CPC的生物相容性和成骨活性。TRO/CMC/Si-CPC具有良好的骨缺损治疗前景,为骨组织工程材料的发展提供了新思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


