Comparative single-cell lineage tracing identifies distinct adipocyte precursor dynamics in skin and inguinal fat
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
White adipose tissue supports essential physiological functions through adipocyte precursor cells (APCs), comprising progenitors and preadipocytes, which generate mature adipocytes during depot expansion. Using single-cell RNA sequencing-based lineage tracing, we characterize APCs in skin adipose tissue—a depot uniquely capable of rapid adipogenesis compared with other sites, such as inguinal adipose. We identify a previously uncharacterized population of immature preadipocytes and reveal distinct differentiation potentials among APCs. Contrary to traditional stepwise differentiation models, progenitors predominantly generate committed preadipocytes, whereas preexisting preadipocytes accumulate in immature states with divergent potential. Leveraging this refined APC hierarchy, we uncover Sox9 as a crucial regulator of progenitor proliferation and adipogenic differentiation. Cross-depot transplantation further demonstrates how intrinsic and extrinsic factors differentially regulate skin progenitor behavior, highlighting distinct adipogenic dynamics between skin and inguinal depots. Together, these insights redefine the cellular hierarchy and molecular mechanisms underpinning rapid adipogenesis in skin adipose tissue.
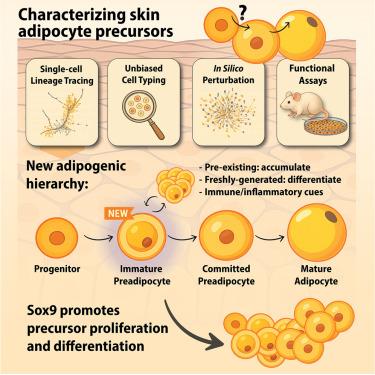
比较单细胞谱系追踪识别皮肤和腹股沟脂肪中不同的脂肪细胞前体动力学
白色脂肪组织通过脂肪细胞前体细胞(APCs)支持基本的生理功能,包括祖细胞和前脂肪细胞,在储存扩张过程中产生成熟的脂肪细胞。使用基于单细胞RNA测序的谱系追踪,我们表征了皮肤脂肪组织中的apc -与其他部位(如腹股沟脂肪)相比,apc具有快速脂肪形成的独特能力。我们确定了一个以前未被表征的未成熟前脂肪细胞群体,并揭示了apc之间不同的分化潜力。与传统的逐步分化模型相反,祖细胞主要产生固定的前脂肪细胞,而先前存在的前脂肪细胞在未成熟状态下积累,具有分化潜能。利用这种完善的APC层次结构,我们发现Sox9是祖细胞增殖和脂肪形成分化的关键调节因子。跨库移植进一步证明了内在和外在因素如何不同地调节皮肤祖细胞的行为,突出了皮肤和腹股沟库之间不同的脂肪形成动力学。总之,这些见解重新定义了支持皮肤脂肪组织快速脂肪形成的细胞层次和分子机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


