Quaternary ammonium salt microspheres loaded with vascular disrupting agents for targeted interventional therapy of hepatocellular carcinoma
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
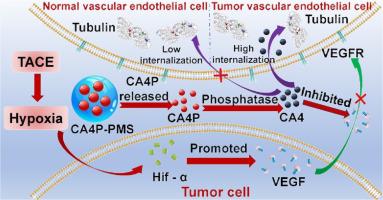
Hepatocellular carcinoma (HCC) is a severe health condition that poses a significant threat to life, characterized by high incidence and mortality rates. Transarterial chemoembolization (TACE) is the primary treatment modality for intermediate and advanced stages of HCC. TACE often fails to completely block all tumor blood supplies, and the hypoxic environment following embolization can lead to neoangiogenesis, negatively impacting the therapeutic efficacy and prognosis of TACE. Combretastatin A4 phosphate (CA4P), as a vascular disrupting agent (VDA), can destroy the tumor's vascular network, inhibit the formation of new blood vessels, and induce ischemic necrosis of the tumor, potentially addressing this issue. Currently, there are no clinically available positively charged microspheres capable of loading CA4P. Based on this, quaternary ammonium salt-based drug-eluting microspheres have been designed to load and sustain the release of CA4P. In vitro and in vivo experiments have demonstrated that CA4P-loaded microsphere therapy can further damage the tumor's vascular system, exacerbate tumor necrosis, and significantly reduce the expression of CD31 and VEGF. This method effectively addresses the current clinical challenge of incomplete tumor blood supply blockage in TACE and the issues of neoangiogenesis caused by the hypoxic environment post-embolization, potentially improving the therapeutic efficacy and prognosis of TACE.
Statement of significance
Hepatocellular carcinoma (HCC) is a severe health condition with high incidence and mortality rates, posing a significant threat to life. Transarterial chemoembolization (TACE) is the primary treatment for intermediate and advanced stages of HCC. However, TACE often fails to completely block all tumor blood supplies, and the hypoxic environment following embolization can lead to neoangiogenesis, negatively impacting therapeutic efficacy and prognosis. To address these limitations, quaternary ammonium salt microspheres were designed to load and sustain the release of combretastatin A4 phosphate (CA4P). CA4P can disrupt tumor vasculature, inhibit new blood vessel formation, and induce ischemic necrosis of the tumor, potentially improving TACE outcomes. This study provides a strategy to enhance TACE efficacy and overcome limitations associated with tumor revascularization.
载血管干扰剂的季铵盐微球用于肝癌靶向介入治疗。
肝细胞癌(HCC)是一种严重的健康状况,对生命构成重大威胁,其特点是发病率和死亡率高。经动脉化疗栓塞(TACE)是中晚期HCC的主要治疗方式。TACE往往不能完全阻断肿瘤的所有血液供应,栓塞后的缺氧环境可导致新生血管生成,对TACE的治疗效果和预后产生负面影响。Combretastatin A4 phosphate (CA4P)作为一种血管破坏剂(vascular disrupting agent, VDA),可以破坏肿瘤的血管网络,抑制新血管的形成,诱导肿瘤的缺血性坏死,有望解决这一问题。目前,临床上还没有能够装载CA4P的带正电微球。在此基础上,设计了季铵盐基药物洗脱微球,以负载和维持CA4P的释放。体外和体内实验表明,负载ca4p的微球治疗可进一步损伤肿瘤血管系统,加剧肿瘤坏死,并显著降低CD31和VEGF的表达。该方法有效解决了目前TACE中肿瘤血供不完全阻断的临床难题,以及栓塞后缺氧环境导致的新生血管生成问题,有望提高TACE的治疗效果和预后。意义声明:肝细胞癌(HCC)是一种发病率和死亡率高的严重疾病,对生命构成重大威胁。经动脉化疗栓塞(TACE)是中晚期HCC的主要治疗方法。然而,TACE往往不能完全阻断肿瘤的所有血液供应,栓塞后的缺氧环境可导致新血管生成,对治疗效果和预后产生负面影响。为了解决这些限制,设计了季铵盐微球来加载和维持磷酸combretastatin A4 (CA4P)的释放。CA4P可破坏肿瘤血管,抑制新血管形成,诱导肿瘤缺血性坏死,可能改善TACE预后。本研究提供了一种提高TACE疗效和克服与肿瘤血运重建相关的局限性的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


