Current status and future perspectives of research on intra-articular drug delivery systems for osteoarthritis therapy
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
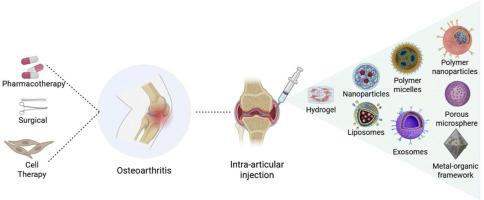
Osteoarthritis (OA) is a degenerative joint disease and the leading cause of joint-related disability worldwide. In current clinical treatment approaches, drug therapy remains widely used, primarily through systemic administration of anti-inflammatory drugs and intra-articular injections. However, effective OA treatment remains challenging due to several barriers: the continuous flow of synovial fluid, the dense and negatively charged cartilage matrix, and the resulting poor drug accumulation at target sites. Moreover, rapid drug clearance from the synovial cavity, frequent dosing requirements, and increased risk of adverse effects further limit treatment efficacy. Recent advancements in intra-articular drug delivery systems, constructed from biological, organic, and inorganic materials, have demonstrated significant potential for OA treatment. Leveraging nanotechnology, these systems not only enhance targeted drug delivery to specific lesions but also enable controlled drug release at inflammatory sites through the optimization of nanocarrier design. This review explores the most innovative strategies for intra-articular drug delivery systems and the key challenges associated with this field. We discuss the development and research progress of emerging delivery technologies, including nanoparticles, liposomes, hydrogels, microspheres, and exosomes. Finally, we highlight the current limitations of intra-articular drug delivery systems and their prospects, aiming to provide valuable insights for further research and clinical translation.
Statement of significance
Osteoarthritis (OA) involves the progressive degradation of articular cartilage components, such as type II collagen and aggrecan, leading to chronic disability. Traditional treatments (systemic drugs, surgery) face limitations like poor targeting, short half-life, and invasiveness. While intra-articular injections enable localized delivery, rapid clearance, cartilage matrix barriers, and inadequate tissue penetration hinder efficacy. Recent advances in nanomaterials and drug delivery systems offer solutions. Stimulus-responsive nanocarriers enable precise targeting and controlled release in inflammatory microenvironments. Nanoparticles, liposomes, hydrogels, and microspheres enhance drug penetration and retention, overcoming rapid clearance. These innovations improve delivery efficiency and prolong the therapeutic effects, thereby advancing cartilage regeneration and personalized treatment. This review examines novel intra-articular delivery strategies to expedite the clinical translation of nanomaterial-based therapies.
骨关节炎关节内给药系统的研究现状及展望
骨关节炎(OA)是一种退行性关节疾病,是世界范围内关节相关残疾的主要原因。在目前的临床治疗方法中,药物治疗仍然被广泛使用,主要是通过全身给药和关节内注射。然而,由于几个障碍,有效的OA治疗仍然具有挑战性:滑液的持续流动,致密且带负电荷的软骨基质,以及由此导致的靶部位药物积累不良。此外,滑膜腔中药物的快速清除、频繁的给药要求和不良反应风险的增加进一步限制了治疗效果。由生物、有机和无机材料构建的关节内药物输送系统的最新进展显示出OA治疗的巨大潜力。利用纳米技术,这些系统不仅增强了对特定病变的靶向药物递送,而且通过优化纳米载体设计,可以在炎症部位控制药物释放。这篇综述探讨了关节内给药系统的最具创新性的策略以及与该领域相关的关键挑战。我们讨论了新兴的递送技术的发展和研究进展,包括纳米颗粒、脂质体、水凝胶、微球和外泌体。最后,我们强调了目前关节内给药系统的局限性及其前景,旨在为进一步的研究和临床转化提供有价值的见解。意义声明:骨关节炎(OA)涉及关节软骨成分(如II型胶原和聚集蛋白)的进行性降解,导致慢性残疾。传统的治疗方法(全身药物、手术)面临着靶向性差、半衰期短和侵入性等局限性。虽然关节内注射可以局部给药,但快速清除、软骨基质障碍和组织渗透不足阻碍了疗效。纳米材料和药物输送系统的最新进展提供了解决方案。刺激反应性纳米载体能够在炎症微环境中精确靶向和控制释放。纳米颗粒,脂质体,水凝胶和微球增强药物渗透和保留,克服快速清除。这些创新提高了输送效率,延长了治疗效果,从而促进了软骨再生和个性化治疗。这篇综述探讨了新的关节内递送策略,以加快纳米材料为基础的治疗的临床转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


