A bioprinted breast cancer model using bioinks of decellularized breast tissue for studying cancer stemness, invasion, and drug efficacy
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
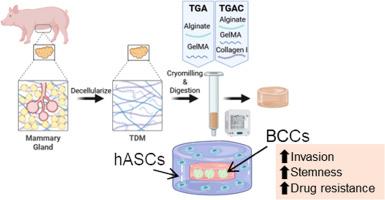
Breast cancer 3D in vitro systems that replicate key tumor characteristics could assist drug discovery by providing more clinically translational models. Breast tumors are formed by hierarchically organized cancer and stromal cells and the extracellular matrix, all of which contribute to the disease progression and treatment response. 3D-bioprinting has enabled the creation of anatomically relevant constructs that better recapitulate the tumor architecture. The extracellular matrix’s role in the tumor outcome has motivated the development of biomimetic bioinks. Among them, bioinks based on decellularized mammary glands can mimic many native biological cues. This work aims to develop a bioprinted 3D in vitro model of breast cancer using a biomimetic bioink based on decellularized mammary glands, and to investigate the effect of this bioink on the malignancy and drug resistance of breast cancer cells. The biomimetic bioink supported cell stemness, invasion, and an immunosuppressive environment but did not promote drug resistance. We also tested the effect of supplementing the bioink with collagen I, which is highly expressed in breast cancer, on breast cancer cells. We observed a higher expression of malignancy markers (COL1A1) and invasion markers (CDH2, MMP2). Next, we bioprinted a cancer model using human adipose mesenchymal stem cells and breast cancer cells to replicate tumor anatomy. These bioprinted models exhibited enhanced resistance to doxorubicin, particularly in the case of the bioink supplemented with collagen I. Therefore, supplementing the bioink with collagen I can promote the creation of more relevant cancer models for drug screening.
Statement of significance
Bioprinted 3D in vitro tumor models are emerging as key tools for drug discovery, as they effectively replicate key tumor characteristics. We developed a bioprinted breast cancer model replicating the tumor structure and extracellular matrix, using a biomimetic bioink based on decellularized mammary glands (TDM) and collagen I. The model consisted of a core of cancer cells surrounded by a layer of mesenchymal stem cells (MSCs). Our findings indicate that TDM-based bioinks enhanced the malignant behavior of the breast cancer cells and promoted the differentiation of MSCs towards cancer-associated fibroblasts. Moreover, cancer cells exhibited increased resistance to chemotherapy in this model, highlighting the importance of mimicking the tumor structure and extracellular matrix in creating more physiologically relevant tumor models.
使用脱细胞乳腺组织生物墨水的生物打印乳腺癌模型,用于研究癌症的干性、侵袭性和药物疗效。
复制关键肿瘤特征的乳腺癌3D体外系统可以通过提供更多的临床转化模型来帮助药物发现。乳腺肿瘤是由分层组织的肿瘤、基质细胞和细胞外基质形成的,所有这些都有助于疾病的进展和治疗反应。3d生物打印已经能够创造出解剖学上相关的结构,更好地概括肿瘤的结构。细胞外基质在肿瘤预后中的作用推动了仿生生物连接的发展。其中,基于去细胞乳腺的生物墨水可以模拟许多天然生物信号。本工作旨在利用基于脱细胞乳腺的仿生生物链接建立生物打印的乳腺癌体外3D模型,并研究该生物链接对乳腺癌细胞恶性和耐药的影响。这种仿生生物链支持细胞的干细胞性、侵袭性和免疫抑制环境,但不促进耐药。我们还测试了补充胶原蛋白I对乳腺癌细胞的影响,胶原蛋白I在乳腺癌中高度表达。我们观察到恶性肿瘤标志物(COL1A1)和侵袭标志物(CDH2, MMP2)的表达较高。接下来,我们使用人类脂肪间充质干细胞和乳腺癌细胞生物打印癌症模型来复制肿瘤解剖结构。这些生物打印模型对阿霉素的耐药性增强,特别是在补充胶原蛋白I的生物链接中,因此,补充胶原蛋白I的生物链接可以促进更相关的癌症模型的创建,用于药物筛选。重要意义:生物打印3D体外肿瘤模型正在成为药物发现的关键工具,因为它们有效地复制了关键的肿瘤特征。我们开发了一个生物打印的乳腺癌模型,复制肿瘤结构和细胞外基质,使用基于脱细胞乳腺(TDM)和胶原i的仿生生物链接。该模型由癌细胞核心组成,周围是一层间充质干细胞(MSCs)。我们的研究结果表明,基于tdm的生物墨水增强了乳腺癌细胞的恶性行为,促进了MSCs向癌症相关成纤维细胞的分化。此外,在该模型中,癌细胞对化疗的耐药性增加,这突出了模拟肿瘤结构和细胞外基质在创建更生理相关的肿瘤模型中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


