A study of electrochemical properties of Fe doped spinel copper cobaltite CuCo2O4 for supercapacitor application
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
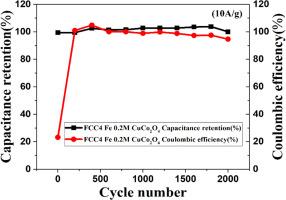
The pursuit of higher energy and power densities in nanomaterials and micromaterials has been the primary cause of the current explosion in supercapacitor research. In this study, spinel pure copper cobaltite CuCo2O4 (CC0) and Fe doped CuCo2O4 electrodes at different mole concentrations (FCC1–0.05 M, FCC2–0.1 M, FCC3–0.15 M, and FCC4–0.2 M) of metal mole complexes are made utilizing the sol-gel procedure using solvents such as citric acid and water. Using Fourier Transform Infrared (FTIR), X-ray diffraction (XRD), Scanning Electron Microscope (SEM), HRTEM, and XPS, the resulting sample is systematically examined to analyze its functional group, crystallite size, shape, and chemical composition. All electrodes are Electric Double Layer Capacitors (EDLCs), according to the Cyclic Voltammetry (CV) test. The Galvanostatic Charge – Discharge (GCD) analysis confirmed that the pure CuCo2O4 (CC0) electrode has a specific capacitance of 80.61F/g at the same current density, while the Fe doped 0.2 M CuCo2O4 (FCC4) electrode has the highest specific capacitance, reaching 163.20F/g at a current density of 1 A/g. Following that, a two-electrode configuration is constructed, such as a Fe doped 0.2 M CuCo2O4 (FCC4) electrode and an activated carbon (AC) electrode. With a specific capacity of 11.62 F/g at a current density of 1 A/g, an energy density of 2.32 Whkg-1, and an impressive power density of 149.99 Wkg-1, the ASC device exhibits outstanding characteristics. The device has a high total capacitive retention value of 99.84 % after 2000 cycles, and supercapacitor devices in particular show remarkable cycle stability. These findings demonstrate that the Fe doped 0.2 M CuCo2O4 (FCC4) electrode has superior electrochemical performance, making it a promising electrode material for supercapacitor applications compared to pure CuCo2O4 (CC0).
铁掺杂尖晶石铜钴酸盐CuCo2O4超级电容器电化学性能研究
在纳米材料和微材料中追求更高的能量和功率密度是当前超级电容器研究爆炸式发展的主要原因。本研究采用溶胶-凝胶法制备了尖晶石纯铜钴酸盐CuCo2O4 (CC0)和Fe掺杂不同摩尔浓度(FCC1-0.05 M、FCC2-0.1 M、FCC3-0.15 M和FCC4-0.2 M)金属摩尔配合物电极,溶剂为柠檬酸和水。利用傅里叶变换红外(FTIR)、x射线衍射(XRD)、扫描电镜(SEM)、HRTEM和XPS对所得样品进行了系统的检测,分析了其官能团、晶体大小、形状和化学成分。根据循环伏安法(CV)测试,所有电极都是双层电电容器(edlc)。恒流充放电(GCD)分析证实,在相同电流密度下,纯CuCo2O4 (CC0)电极的比电容为80.61F/g,而掺铁0.2 M的CuCo2O4 (FCC4)电极的比电容最高,在电流密度为1 a /g时达到163.20F/g。然后,构建了一个双电极结构,即Fe掺杂0.2 M CuCo2O4 (FCC4)电极和活性炭(AC)电极。ASC器件在电流密度为1 a /g时的比容量为11.62 F/g,能量密度为2.32 Wkg-1,功率密度为149.99 Wkg-1,具有突出的特性。该器件在2000次循环后总电容保持值高达99.84%,特别是超级电容器器件表现出显著的循环稳定性。这些发现表明,与纯CuCo2O4 (CC0)相比,掺铁0.2 M CuCo2O4 (FCC4)电极具有优越的电化学性能,是一种很有前景的超级电容器电极材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


