Mechanistic insights into dye adsorption on chitosan-functionalized bentonite: synergizing experiments and computational study
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
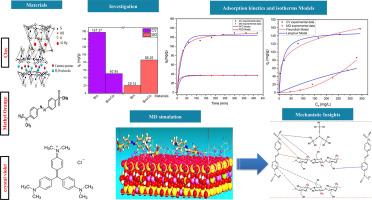
Natural Moroccan bentonite (Bnt) exhibited a high adsorption capacity for crystal violet (CV, a cationic dye), reaching 157.37 mg/g. Conversely, its affinity for methyl orange (MO, an anionic dye) was limited (20.14 mg/g). However, the synthesized bentonite-chitosan composite (Bnt-Cs) features protonated amine groups, which enhance electrostatic and hydrogen-bond interactions, increasing the adsorption of MO dye by 76.65%. The kinetic data revealed that MO adsorption followed the pseudo-first-order (PFO) model, whereas CV adsorption was better described by the pseudo-second-order (PSO) model. These two models are governed by distinct diffusion mechanisms. Moreover, the adsorption isotherms for both dyes aligned well with the Freundlich model. Additionally, the Density Functional Theory (DFT) calculations indicated that CV’s narrower HOMO–LUMO gap and higher molecular softness were consistent with its enhanced reactivity and stronger interaction with the adsorbent. Furthermore, the molecular dynamics (MD) simulations confirmed the spontaneous, physically driven nature of the adsorption process. The close agreement between computational predictions and experimental data provides robust validation for the proposed adsorption mechanisms, offering clear mechanistic insights into dye adsorption processes.
壳聚糖功能化膨润土对染料吸附的机理:协同实验和计算研究
天然摩洛哥膨润土(Bnt)对结晶紫(阳离子染料CV)具有较高的吸附量,吸附量可达157.37 mg/g。相反,其对甲基橙(MO,阴离子染料)的亲和力有限(20.14 mg/g)。然而,合成的膨润土-壳聚糖复合材料(Bnt-Cs)具有质子化胺基团,增强了静电和氢键相互作用,使MO染料的吸附性提高了76.65%。动力学数据表明,MO吸附符合准一阶(PFO)模型,而CV吸附符合准二阶(PSO)模型。这两种模型由不同的扩散机制控制。此外,两种染料的吸附等温线与Freundlich模型一致。此外,密度泛函理论(DFT)计算表明,CV具有更窄的HOMO-LUMO间隙和更高的分子柔软度,这与其增强的反应性和与吸附剂的更强相互作用是一致的。此外,分子动力学(MD)模拟证实了吸附过程的自发、物理驱动性质。计算预测和实验数据之间的密切一致为提出的吸附机制提供了强有力的验证,为染料吸附过程提供了清晰的机理见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


