Cilostazol-loaded acellular vascular graft for suppressing intimal hyperplasia
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-07-28
DOI:10.1016/j.bioadv.2025.214433
引用次数: 0
Abstract
Local vascular injury caused by vascular transplantation and endarterectomy causes intimal hyperplasia accompanied by matrix degradation by the matrix metalloproteinase (MMP) and proliferation of acute smooth muscle cells (SMCs) during the wound healing. In small vessels, excessive intimal thickening easily induces graft occlusion, and regulation of these local events is critical for graft patency. Although cilostazol (CLZ) has been investigated in clinical trials as a stenosis-suppressing medicine, strategies targeting the suppression of anastomotic stenosis are insufficient. In this study, we developed CLZ-loaded acellular grafts that respond to MMP using a reprecipitation process. CLZ weighing 112 μg was loaded into an acellular graft (CLZ-Graft) weighing 10 mg via reprecipitation. The loaded CLZ was gradually released via matrix degradation but did not leak without degradation. The SMC phenotype changed to a contracted type and proliferation was suppressed when the SMCs were cultured with the CLZ-Graft lysate. The lysate did not inhibit endothelial cell migration or vasculogenesis. In a rat carotid artery ablation model, the graft diameter decreased to 48 % under control conditions. When the CLZ-Graft was placed around the injured carotid artery in rats, acute stenosis was suppressed to only 31 % and 18 % at two and four weeks, respectively. These results indicated that intimal hyperplasia was effectively suppressed by the local release of CLZ from CLZ-Graft in response to MMP. These findings demonstrate that drug-releasing from the decellularized tissue that responds to MMP is an effective strategy for inhibiting vascular stenosis.
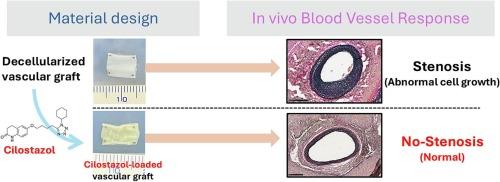
西洛他唑负载的脱细胞血管移植物抑制内膜增生
血管移植和动脉内膜切除术引起的局部血管损伤,在创面愈合过程中,伴随着基质金属蛋白酶(MMP)的降解和急性平滑肌细胞(SMCs)的增殖,导致内膜增生。在小血管中,过度的内膜增厚容易导致移植物闭塞,对这些局部事件的调节对移植物通畅至关重要。尽管西洛他唑(cilostazol, CLZ)作为一种抑制狭窄的药物已经在临床试验中进行了研究,但针对吻合口狭窄的抑制策略还不够。在这项研究中,我们开发了clz负载的脱细胞移植物,使用再沉淀过程对MMP有反应。重112 μg的CLZ通过再沉淀加载到重10 mg的无细胞移植物(CLZ- graft)中。负载的CLZ通过基质降解逐渐释放,但没有降解而不泄漏。用CLZ-Graft裂解液培养SMCs后,SMCs表型转变为收缩型,增殖受到抑制。裂解液不抑制内皮细胞迁移或血管生成。在大鼠颈动脉消融模型中,对照条件下移植物直径减小到48%。当CLZ-Graft被放置在损伤的大鼠颈动脉周围时,在第2周和第4周,急性狭窄分别被抑制到31%和18%。这些结果表明,CLZ- graft在MMP作用下局部释放CLZ可以有效抑制内膜增生。这些发现表明,从对MMP有反应的脱细胞组织中释放药物是抑制血管狭窄的有效策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


