Impact of rotating night shift work on immune homeostasis among hospital employees during the COVID-19 pandemic
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Background
Night shifts may disrupt circadian rhythms and immunity, potentially leading to health issues such as increased susceptibility to infectious diseases and impaired vaccine responses. This concern is particularly relevant for hospital employees during the COVID-19 pandemic. Our study aims to investigate the immune homeostasis of hospital workers engaged in rotating night shifts during the pandemic.
Methods
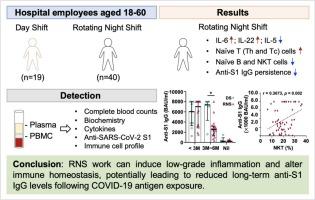
Healthy hospital employees aged 18–60 were enrolled and divided into two groups: the day shift (DS) group (n = 19) and the rotating night shift (RNS) group (n = 40). Blood samples were collected for analysis, including complete blood count, biochemistry, cytokines, anti-SARS-CoV-2 S1 antibody (anti-S1 IgG) titers, and immune cell subsets.
Results
The RNS group exhibited skewed Th1 immunity, characterized by significant elevations in IL-6 and IL-22, increasing trends in TNF-α and the IFN-γ/IL-5 ratio, along with a significant reduction in IL-5. Both groups demonstrated comparable rates of COVID-19 infection, and all participants showed effectively boosted anti-S1 IgG titers following antigen exposure (natural infection or vaccination). However, the RNS had lower anti-S1 IgG titers 3 to 6 months after antigen exposure. Additionally, decreased proportions of naïve B, and NKT cells along with an increase in T cell subsets, were detected in the RNS group. A significant positive correlation was also found between anti-S1 IgG titers and the proportion of NKT cells.
Conclusion
These findings suggest that RNS work induces low-grade inflammation and disrupts immune homeostasis, possibly lowering long-term anti-S1 IgG level after COVID-19 antigen exposure.
COVID-19大流行期间夜班轮班对医院员工免疫稳态的影响
夜班可能会扰乱昼夜节律和免疫力,可能导致健康问题,如对传染病的易感性增加和疫苗反应受损。在COVID-19大流行期间,这一担忧对医院员工尤为重要。我们的研究旨在调查大流行期间从事夜班的医院工作人员的免疫稳态。方法选取18 ~ 60岁健康的医院职工为研究对象,分为白班组(DS) 19例和夜班轮换组(RNS) 40例。采集血样进行分析,包括全血细胞计数、生化、细胞因子、抗sars - cov -2 S1抗体(抗S1 IgG)滴度和免疫细胞亚群。结果RNS组Th1免疫偏斜,表现为IL-6和IL-22显著升高,TNF-α和IFN-γ/IL-5比值升高,IL-5显著降低。两组的COVID-19感染率相当,所有参与者在抗原暴露(自然感染或接种疫苗)后,抗s1 IgG滴度都有效提高。然而,抗原暴露后3至6个月,RNS的抗s1 IgG滴度降低。此外,在RNS组中检测到naïve B和NKT细胞的比例下降以及T细胞亚群的增加。抗s1 IgG滴度与NKT细胞比例呈显著正相关。结论RNS工作诱导低级别炎症,破坏免疫稳态,可能降低COVID-19抗原暴露后的长期抗s1 IgG水平。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


