Molecular insights into CYP19A1 mutations and their role in estrogen production
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
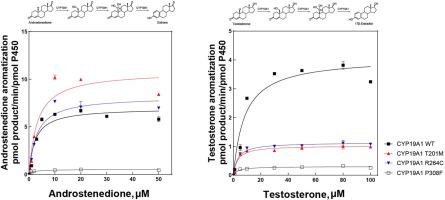
Human cytochrome P450 enzyme CYP19A1, commonly referred to as aromatase, plays a critical role in estrogen biosynthesis by catalyzing the conversion of C19 androgens into aromatic C18 estrogens. Alterations in aromatase activity have been implicated in the development and progression of estrogen-dependent diseases. Genetic mutations, including nonsynonymous single nucleotide polymorphisms (SNPs), can markedly impact the catalytic function of CYP19A1. In this study, we investigated the functional implications of six CYP19A1 variants (R192H, R192Q, T201M, R264C, P308F, and M364T). These variants were generated via site-directed mutagenesis, expressed in Escherichia coli, and purified. The T201M, R264C, and P308F variants exhibited measurable expression levels ranging from 90 to 150nmol P450 per liter culture, whereas no detectable P450 holoenzyme was observed for the R192H, R192Q, and M364T variants. Spectral binding assays revealed typical type I spectral shifts upon androgen binding for all purified variants with tight affinity. Catalytic activities of testosterone and androstenedione aromatization were assessed by UPLC–mass spectrometry, and steady-state kinetic analyses demonstrated that T201M and R264C retained catalytic efficiencies comparable to wild-type, with efficiency ratios of 1.1–0.9 for androstenedione and 0.6–0.8 for testosterone, respectively, relative to the wild-type. In contrast, P308F showed marked reductions in catalytic efficiencies (0.2 and 0.1), driven by decreases in both kcat and Km values. Structural analysis indicates that the proline residue at position 308, situated within the I-helix, is likely critical for stabilizing a catalytically active conformation of the enzyme-substrate complex. These results provide valuable insights into the functional role of CYP19A1 in estrogen biosynthesis and could inform the design of innovative therapies for estrogen-related diseases.
CYP19A1突变及其在雌激素产生中的作用
人类细胞色素P450酶CYP19A1,通常被称为芳香化酶,在雌激素的生物合成中起关键作用,催化C19雄激素转化为芳香型C18雌激素。芳香化酶活性的改变与雌激素依赖性疾病的发生和进展有关。基因突变,包括非同义单核苷酸多态性(SNPs),可以显著影响CYP19A1的催化功能。在这项研究中,我们研究了6种CYP19A1变异(R192H、R192Q、T201M、R264C、P308F和M364T)的功能影响。这些变异是通过定点诱变产生的,在大肠杆菌中表达,并纯化。T201M、R264C和P308F突变体每升培养物中P450的表达量在90 - 150nmol之间,而R192H、R192Q和M364T突变体中没有检测到P450全酶。光谱结合分析显示,所有纯化的具有紧密亲和力的变异在雄激素结合时具有典型的I型光谱偏移。通过uplc质谱法测定了T201M和R264C对睾酮和雄烯二酮芳构化的催化活性,稳态动力学分析表明,T201M和R264C的催化效率与野生型相当,相对于野生型,其对雄烯二酮的效率分别为1.1-0.9和0.6-0.8。相比之下,P308F的催化效率明显降低(0.2和0.1),这是由于kcat和Km值的降低所致。结构分析表明,位于i -螺旋内308位的脯氨酸残基可能对稳定酶-底物复合物的催化活性构象至关重要。这些结果为CYP19A1在雌激素生物合成中的功能作用提供了有价值的见解,并可能为设计雌激素相关疾病的创新疗法提供信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


