Metabolic modulation-driven self-reinforcing pyroptosis-STING nanoadjuvant for potentiated metalloimmunotherapy
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Pyroptosis is a critical process that triggers inflammatory responses and mitochondrial DNA (mtDNA) release, thereby activating the cGAS-STING pathway. However, tumor metabolism, particularly glycolysis, often suppresses immune activation. To address this, we developed GOCoF2, a self-amplifying pyroptosis-STING nanoadjuvant that integrates glucose oxidase (GOx) with cobalt fluoride (CoF2) nanoenzymes. This nanoadjuvant excelled in converting intratumoral H2O2 into reactive oxygen species (ROS), inducing cell pyroptosis. Its self-sustaining mechanism involved glucose depletion and continuous H2O2 generation, ensuring persistent catalytic activity. This metabolic manipulation and induction of oxidative stress significantly enhance pyroptosis in tumor cells. The released mtDNA subsequently activated the cGAS-STING pathway, with Co2+ further amplifying this effect. Notably, glucose-dependent TREX2 inhibition intensified cGAS-STING activation through metabolic regulation, leading to a strong immune response and tumor growth suppression. When combined with immune checkpoint blockade therapy, GOCoF2 significantly inhibited primary and distant tumor progression via systemic immune activation. Additionally, we formulated GOCoF2-lipiodol for transarterial embolization, which demonstrated superior efficacy in a rat model of orthotopic hepatocellular carcinoma. This study not only sheds light on the intricate relationship between tumor metabolism and immune regulation but also introduces a novel therapeutic approach for hepatocellular carcinoma.
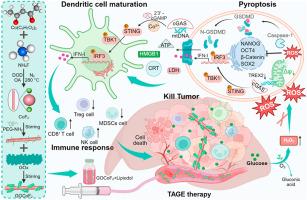
代谢调节驱动的自我强化热分解- sting纳米佐剂用于增强金属免疫治疗
焦亡是触发炎症反应和线粒体DNA (mtDNA)释放的关键过程,从而激活cGAS-STING通路。然而,肿瘤代谢,特别是糖酵解,经常抑制免疫激活。为了解决这个问题,我们开发了GOCoF2,一种自扩增的焦解毒- sting纳米佐剂,将葡萄糖氧化酶(GOx)与氟化钴(CoF2)纳米酶结合在一起。这种纳米佐剂擅长将肿瘤内的H2O2转化为活性氧(ROS),诱导细胞焦亡。其自我维持机制包括葡萄糖消耗和持续的H2O2生成,确保持续的催化活性。这种代谢操纵和氧化应激的诱导显著增强了肿瘤细胞的焦亡。释放的mtDNA随后激活了cGAS-STING途径,而Co2+进一步放大了这一作用。值得注意的是,葡萄糖依赖性TREX2抑制通过代谢调节增强了cGAS-STING的激活,从而导致强烈的免疫应答和肿瘤生长抑制。当与免疫检查点阻断治疗联合使用时,GOCoF2通过全身免疫激活显着抑制原发性和远处肿瘤的进展。此外,我们配制了gocof2 -脂醇用于经动脉栓塞,在大鼠原位肝细胞癌模型中显示出优越的疗效。该研究不仅揭示了肿瘤代谢与免疫调节之间的复杂关系,而且为肝癌的治疗提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


