Effect of hydrophobic character of diammonium-capping agents for the synthesis of nanocrystalline MOR zeolite
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Multiammonium surfactants are known to act as effective capping agents in the synthesis of zeolite nanoparticles. While the attractive interactions between ammonium head groups and zeolite surfaces play a critical role in capping, the influence of the surfactant tail structures remains poorly understood. In this study, a series of diammonium surfactants with different tail lengths (i.e., CnH2n+1-N+(CH3)2-C6H12-N+(CH3)2-CmH2m+1, 4 ≤ n, m ≤ 22) were systematically tested to investigate the effect of hydrophobic character on the morphology of mordenite (MOR) zeolite nanocrystals. Capping efficiency was assessed using X-ray diffraction, nitrogen adsorption-desorption isotherms, and scanning electron microscopy. The results showed that surfactants with a moderate hydrophilic/hydrophobic balance—quantified by the carbon-to-nitrogen (C/N+) ratio—exhibited the highest capping efficiency, resulting in smaller particle sizes and larger external surface areas. Furthermore, non-Gemini surfactants (i.e., n ≠ m) demonstrated significantly enhanced capping efficiency, compared to symmetric Gemini surfactants (i.e., n = m). These findings offer valuable insights into the molecular design of surfactants in the controlled synthesis of zeolite nanoparticles.
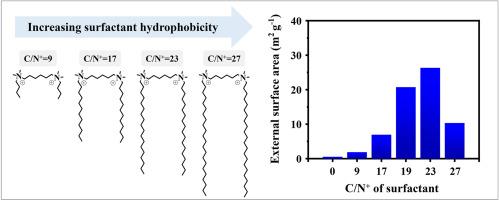
包覆剂疏水特性对纳米晶MOR分子筛合成的影响
在沸石纳米颗粒的合成中,多铵表面活性剂被认为是有效的封盖剂。虽然铵头基团与沸石表面之间的相互吸引作用在封盖中起着关键作用,但表面活性剂尾部结构的影响仍然知之甚少。本研究系统测试了一系列尾长不同的二铵表面活性剂(CnH2n+1- n+ (CH3)2-C6H12-N+(CH3)2-CmH2m+ 1,4≤n, m≤22),研究疏水特性对丝光沸石纳米晶形貌的影响。利用x射线衍射、氮吸附-解吸等温线和扫描电镜对封盖效率进行了评估。结果表明,以碳氮比(C/N+)衡量,具有中等亲疏水平衡的表面活性剂表现出最高的封盖效率,从而产生更小的粒径和更大的外表面积。此外,与对称Gemini表面活性剂(n = m)相比,非Gemini表面活性剂(即n≠m)的封井效率显著提高。这些发现为沸石纳米颗粒受控合成中表面活性剂的分子设计提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


