Theoretical study on the co-adsorption effect of H atoms on NH3 decomposition over Pt(100) and Pt(111) surfaces
IF 5
Q2 ENERGY & FUELS
引用次数: 0
Abstract
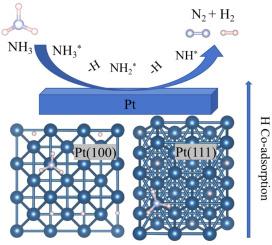
The influence of co-adsorbed hydrogen (H) atoms on the decomposition of ammonia (NH3) over two representative crystalline facets of platinum catalyst, i.e., Pt(100) and Pt(111), is investigated using density functional theory (DFT) and microkinetic modeling. The results reveal that NH₃ preferentially adsorbs on top sites, with its binding strength decreasing at higher coverages due to intermolecular repulsion. Although NH3 adsorption is progressively unfavored on both facets with increased coverage by co-adsorbed H atoms, facet-dependent effects are observed: while Pt(100) maintains stable NH3 decomposition energetics even at high H coverages, Pt(111) shows significant inhibition effect with increased reaction barriers and destabilized intermediates. Microkinetic simulations further confirm that Pt(100) exhibits superior catalytic activity, particularly in N–N coupling and N2 desorption, compared to Pt(111). These findings highlight the critical role of surface structure and hydrogen coverage in modulating NH3 decomposition kinetics, providing insights for optimizing Pt-based catalysts in NH3-based energy systems.
H原子对Pt(100)和Pt(111)表面NH3分解共吸附效应的理论研究
利用密度泛函理论(DFT)和微动力学模型研究了共吸附氢(H)原子对铂催化剂Pt(100)和Pt(111)两个代表性晶面上氨(NH3)分解的影响。结果表明,NH₃优先吸附在顶部位点上,由于分子间排斥,它的结合强度在更高的覆盖率下降低。尽管随着共吸附H原子覆盖率的增加,NH3的吸附在两个方面都逐渐变得不利,但观察到面依赖效应:即使在高H覆盖率下,Pt(100)也能保持稳定的NH3分解能量,而Pt(111)随着反应障碍和不稳定中间体的增加而表现出显著的抑制作用。微动力学模拟进一步证实,与Pt(111)相比,Pt(100)表现出更好的催化活性,特别是在N-N耦合和N2脱附方面。这些发现强调了表面结构和氢覆盖在调节NH3分解动力学中的关键作用,为优化NH3能源体系中基于pt的催化剂提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


