Exploring airway biological barriers: Implications for IgG-based therapeutics in respiratory infections
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-07-25
DOI:10.1016/j.ejpb.2025.114817
引用次数: 0
Abstract
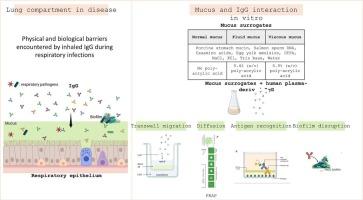
Despite the considerable value of prophylactic vaccines and post-exposure antimicrobials, respiratory infections represent a huge burden. The control of bacterial respiratory infections is compromised by the alarming rise in antibiotic resistance, which underscores the requirement for alternative or complementary interventions. Recent studies have demonstrated that anti-infective IgG-based therapeutics can have an important role in tackling pathogens, and that inhalation is a promising route to target respiratory infections in the lung. Here, we investigated the impact of physical and biological barriers encountered by inhaled IgG during respiratory infections. These include hyper-production and thickening of mucus, as well as bacteria embedded in biofilm. Experiments using artificial bronchiectasis-like mucus showed reduced mobility and pathogen-binding of IgG as compared to artificial mucus with healthy characteristics. Our findings highlight the reduced ability of IgG to effectively opsonize pathogens and trigger effector functions during respiratory infections, such as the ones associated to bronchiectasis. In parallel, IgG was able to disrupt P.aeruginosa biofilm integrity upon repeat administrations in vitro. These results support the use of locally applied human polyclonal IgG in infection-driven chronic lung diseases and highlight the challenges to consider for the development of inhaled IgG.
探索气道生物屏障:基于igg的呼吸道感染治疗的意义
尽管预防性疫苗和接触后抗微生物药物具有相当大的价值,但呼吸道感染是一个巨大的负担。细菌呼吸道感染的控制因抗生素耐药性的惊人上升而受到损害,这突出表明需要采取替代或补充干预措施。最近的研究表明,抗感染的基于igg的治疗方法可以在解决病原体方面发挥重要作用,并且吸入是一种有希望的途径来靶向肺部呼吸道感染。在这里,我们研究了呼吸道感染期间吸入IgG遇到的物理和生物屏障的影响。这些包括粘液的过量产生和增厚,以及嵌入生物膜的细菌。实验显示,与健康的人工粘液相比,人工支气管扩张样粘液的流动性和病原体结合性降低。我们的研究结果强调了IgG在呼吸道感染(如与支气管扩张相关的感染)期间有效调节病原体和触发效应功能的能力降低。同时,IgG能够在体外重复给药后破坏铜绿假单胞菌生物膜的完整性。这些结果支持在感染驱动的慢性肺部疾病中使用局部应用的人多克隆IgG,并强调了开发吸入IgG需要考虑的挑战。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


