Molecular diversity of three-component reaction of alkyl isocyanides, dialkyl but-2-ynedioates and 2-arylidene-1,3-indanediones
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
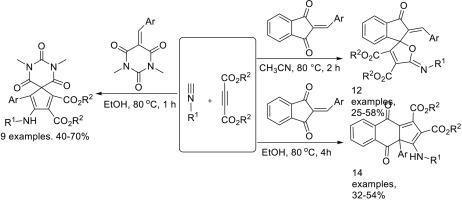
The three-component reaction of alkyl isocyanides, dialkyl but-2-ynedioates and 2-arylidene-1,3-indanediones showed very interesting molecular diversity. The three-component reaction in refluxing acetonitrile afforded functionalized spiro[furan-2,1′-indene] derivatives. On the other hand, the three-component reaction in refluxing ethanol gave functionalized cyclopenta[b]naphthalene derivatives. In addition, the similar three-component reaction of alkyl isocyanides, dialkyl but-2-ynedioates and 5-arylidene-N,N′-dimethylbarbituric acids resulted in functionalized diazaspiro[4.5]deca-1,3-diene-1,2-dicarboxylates in satisfactory yields. A plausible reaction mechanism including in situ of Huisgen's 1,4-dipole, and sequential annulation reaction was proposed to explain the formation of the different polycyclic compounds.
烷基异氰酸酯、二烷基丁-2-炔酸酯和2-芳基-1,3-茚二酮三组分反应的分子多样性
烷基异氰酸酯、二烷基丁-2-炔酸酯和2-芳基-1,3-茚二酮的三组分反应显示出非常有趣的分子多样性。三组分反应在回流乙腈中得到功能化的螺[呋喃-2,1 ' -茚]衍生物。另一方面,三组分反应在回流乙醇中得到功能化环五[b]萘衍生物。此外,烷基异氰酸酯、二烷基丁-2-炔酸酯和5-芳基烯-N,N ' -二甲基巴比妥酸的类似三组分反应可得到功能化的重氮斯匹罗[4.5]十二-1,3-二烯-1,2-二羧酸酯,收率令人满意。提出了一种合理的反应机制,包括Huisgen的1,4偶极子原位反应和顺序环化反应,以解释不同多环化合物的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


