Effects of acid-mediated deamination of cytidine and 5-methylcytidine on solid-phase synthesis of oligonucleotides
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Oligonucleotide therapeutics have attracted considerable attention in recent years as a new drug discovery modality. They are generally produced via solid-phase oligonucleotide synthesis based on phosphoramidite chemistry. Oligonucleotide therapeutics form Watson–Crick base pairs with the target RNA and strictly recognize their sequence for gene regulation; therefore, structural changes in nucleobases could be crucial and should be avoided during oligonucleotide synthesis. In this study, we investigated the deamination of cytidine and 5-methylcytidine during the acid-mediated removal of dimethoxytrityl (DMTr) group of solid-phase oligonucleotide synthesis. Our results suggest that the deamination reaction proceeds regardless of the structure of the sugar moiety of the phosphoramidite monomer, particularly in reaction systems containing more than 1 % of water. The data from this study is believed highly beneficial and helpful for avoiding acid-mediated deamination of cytidine and 5-methylcytidine during oligonucleotide synthesis.
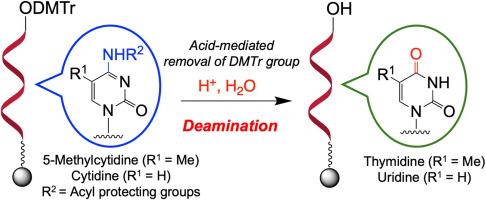
酸介导胞苷和5-甲基胞苷脱胺对固相合成寡核苷酸的影响
寡核苷酸疗法作为一种新的药物发现方式近年来引起了人们的广泛关注。它们通常是通过基于酰胺磷化学的固相寡核苷酸合成来生产的。寡核苷酸疗法与靶RNA形成沃森-克里克碱基对,并严格识别其序列进行基因调控;因此,在寡核苷酸合成过程中,核碱基的结构变化可能是至关重要的,应该避免。在本研究中,我们研究了固相寡核苷酸合成中酸介导去除二甲氧基三硝基(DMTr)时胞苷和5-甲基胞苷的脱胺反应。我们的研究结果表明,脱氨反应的进行与磷酸酰胺单体的糖部分的结构无关,特别是在含有超过1%的水的反应体系中。本研究的数据被认为是非常有益的,有助于避免在寡核苷酸合成过程中酸介导的胞苷和5-甲基胞苷脱胺。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


