Ingestible optoelectronic capsules enable bidirectional communication with engineered microbes for controllable therapeutic interventions
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
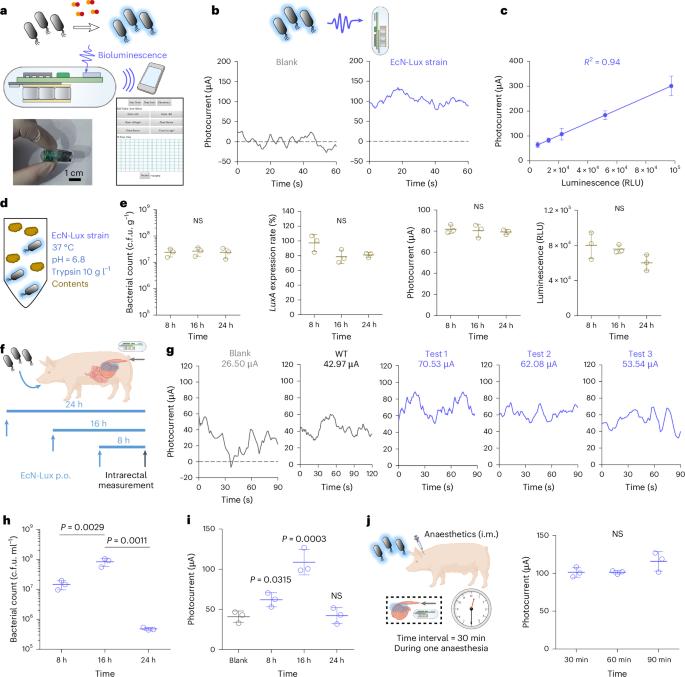
Engineered microbes can be used for biomolecular sensing and therapeutic interventions. However, they cannot be monitored and controlled while in vivo. Here we combine optogenetically engineered Escherichia coli Nissle 1917, an ingestible optoelectronic capsule and a wireless smartphone to establish a bidirectional biological–optical–electronic signal processing chain for diagnostic or therapeutic capabilities under user control. As a proof of concept, we engineered E. coli Nissle 1917 to detect inflammation-associated nitric oxide in the pig gut and generate a bioluminescent signal for diagnosis of colitis. This signal is transduced by the optoelectronic capsule into a wireless electrical signal and remotely monitored by a smartphone. Smartphone wireless signals activate LED irradiation in the optoelectronic capsule, in turn activating the microbial expression and secretion of an anti-inflammatory nanobody to alleviate colitis in pigs. This approach highlights the potential for integrating synthetic biology and optoelectronics for digital health monitoring and controllable intervention. A smartphone-controlled ingestible capsule can have a two-way communication with engineered microbes inside the gut via light-induced signals.
可摄取的光电胶囊能够与工程微生物进行双向通信,以实现可控的治疗干预
工程微生物可用于生物分子传感和治疗干预。然而,它们在体内无法被监测和控制。在这里,我们将光基因工程的大肠杆菌Nissle 1917,一种可消化的光电胶囊和无线智能手机结合起来,建立了一个双向的生物-光学-电子信号处理链,用于用户控制的诊断或治疗能力。作为概念验证,我们设计了大肠杆菌Nissle 1917来检测猪肠道中与炎症相关的一氧化氮,并产生用于诊断结肠炎的生物发光信号。该信号由光电胶囊转换为无线电信号,并由智能手机远程监控。智能手机无线信号激活光电胶囊中的LED照射,进而激活抗炎纳米体的微生物表达和分泌,以减轻猪的结肠炎。这种方法强调了将合成生物学和光电子学集成到数字健康监测和可控干预中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


