Peptidoglycan–outer membrane attachment generates periplasmic pressure to prevent lysis in Gram-negative bacteria
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Bacteria are subject to a substantial concentration differential of osmolytes between the interior and exterior of the cell, resulting in turgor pressure. Failure to mechanically balance this turgor pressure causes cells to burst. Here, using microfluidics, imaging, biochemistry and mathematical modelling, we analysed how Escherichia coli cells with structural mutations in the envelope respond to hypoosmotic shocks. We show that the peptidoglycan cell wall forms a mechanical unit with the outer membrane that limits periplasmic volume increase under hypoosmotic shock, allowing osmotic pressure build-up in the periplasm. In turn, this periplasmic pressure balances cytoplasmic turgor across the inner membrane, preventing cell lysis and death. Thus, while the peptidoglycan layer is necessary, it is not sufficient to maintain turgor and protect cells from lysis. We propose a model in which the entire cell envelope, including the periplasm, collectively enables Gram-negative bacteria to overcome osmotic challenges. Outer membrane attachment to peptidoglycan enables periplasmic pressure to build up and counter cytoplasmic turgor pressure, preventing lysis during osmotic challenges in Escherichia coli.
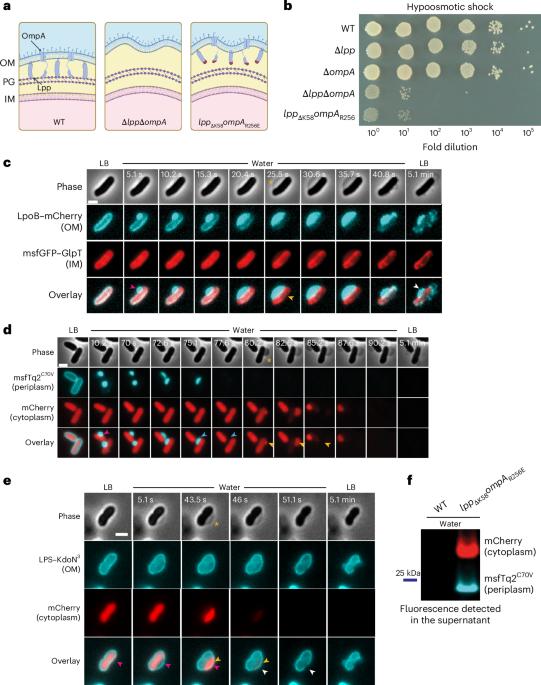

在革兰氏阴性菌中,肽聚糖-外膜附着产生质周压力以防止裂解
细菌受到细胞内部和外部渗透液的巨大浓度差的影响,导致胀压。机械平衡这种膨胀压力的失败会导致细胞破裂。在这里,利用微流体、成像、生物化学和数学模型,我们分析了包膜结构突变的大肠杆菌细胞对低渗冲击的反应。我们发现肽聚糖细胞壁与外膜形成一个机械单元,在低渗休克下限制周质体积的增加,允许周质中渗透压的积聚。反过来,这种质周压力平衡细胞膜上的细胞质膨胀,防止细胞裂解和死亡。因此,虽然肽聚糖层是必需的,但它不足以维持肿胀和保护细胞免受裂解。我们提出了一个模型,其中整个细胞包膜,包括外周质,共同使革兰氏阴性菌克服渗透挑战。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


