Low temperature thermolysis and hydrolysis of MgH2 generated from a titanium-mediated hydrogenation of Mg2Si
IF 13.8
1区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
The magnesium-silicon hydrogen storage system (Mg2Si+H2↔MgH2+Si) has attracted considerable attention with the potential of achieving near-room temperature hydrogen sorption since the reaction enthalpy is only ∼36.8 kJ/mol H2, offering much better thermodynamic properties over MgH2. However, the rehydrogenation of Mg2Si faces significant kinetic barriers, restricting its practical utilization. In this work, hydrogen-assisted high-energy ball milling (HHBM) was employed to hydrogenate Mg2Si. XRD and HRTEM results confirmed that Mg2Si was successfully converted into MgH2 under the influence of titanium (Ti), achieving a conversion rate up to 76%. Aberration-corrected TEM (AC-TEM) revealed that in situ formed MgH2 and titanium silicide (TiSi2) were homogenously mixed with robust interfaces. Such a growth mode reduces the migration path of Si atoms and the stability of Mg2Si, thus promoting the formation of MgH2. Furthermore, the as-synthesized MgH2 particles with ultrafine particle size and adjacent catalysts show superior thermolysis and hydrolysis performances. Consequently, the composite exhibits excellent hydrogen storage properties, absorbing 50% of its total hydrogen capacity at room temperature and initiating dehydrogenation at 114.3 °C. In situ synchrotron X-ray diffraction (ISXRD) confirms the dehydrogenation of in situ generated ultrafine MgH2 and part of TiSi2 is converted to Mg2Si at 283 °C. In addition, the ultrafine, defect-rich MgH2 particles show favorable hydrolysis kinetics and high conversion efficiency at relatively low temperatures (91.9% of the hydrogen yield can be achieved at 5 °C). This method developed in this work exhibits a new way to synthesize high-performance hydrogen storage materials.
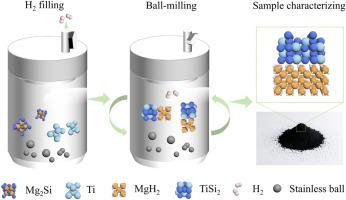
钛介导Mg2Si加氢生成MgH2的低温热解和水解
镁硅储氢系统(Mg2Si+H2↔MgH2+Si)由于其反应焓仅为~ 36.8 kJ/mol H2,具有比MgH2更好的热力学性质,因此具有实现近室温吸氢的潜力,引起了相当大的关注。然而,Mg2Si的再加氢存在明显的动力学障碍,制约了其实际应用。本文采用氢辅助高能球磨(HHBM)对Mg2Si进行了氢化处理。XRD和HRTEM结果证实,在钛(Ti)的作用下,Mg2Si成功转化为MgH2,转化率高达76%。像差校正透射电镜(AC-TEM)显示原位形成的MgH2和硅化钛(TiSi2)均匀混合,界面坚固。这种生长方式减少了Si原子的迁移路径和Mg2Si的稳定性,从而促进了MgH2的形成。此外,合成的粒径超细的MgH2颗粒和邻近的催化剂表现出优异的热解和水解性能。因此,复合材料表现出优异的储氢性能,在室温下吸收其总氢容量的50%,并在114.3℃下引发脱氢。原位同步x射线衍射(ISXRD)证实了原位生成的超细MgH2脱氢,部分TiSi2在283℃下转化为Mg2Si。此外,超细、富含缺陷的MgH2颗粒在较低温度下表现出良好的水解动力学和较高的转化效率(5℃时可达到91.9%的产氢率)。该方法为合成高性能储氢材料提供了一条新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Magnesium and Alloys
Engineering-Mechanics of Materials
CiteScore
20.20
自引率
14.80%
发文量
52
审稿时长
59 days
期刊介绍:
The Journal of Magnesium and Alloys serves as a global platform for both theoretical and experimental studies in magnesium science and engineering. It welcomes submissions investigating various scientific and engineering factors impacting the metallurgy, processing, microstructure, properties, and applications of magnesium and alloys. The journal covers all aspects of magnesium and alloy research, including raw materials, alloy casting, extrusion and deformation, corrosion and surface treatment, joining and machining, simulation and modeling, microstructure evolution and mechanical properties, new alloy development, magnesium-based composites, bio-materials and energy materials, applications, and recycling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


