Excessive ultra-processed foods exposure aggravates ulcerative colitis via macrophage ferroptosis
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Ulcerative colitis (UC), a chronic inflammatory disease with global prevalence, is increasingly associated with environmental exposure to modern dietary hazards, particularly ultra-processed foods containing oxidized phytosterols. While industrial food processing techniques and repeated oil reuse are known to amplify formation of phytosterol oxidation products, their biological impacts on intestinal inflammation remain unexplored. This study focuses on 7-ketositosterol (KS), the most abundant phytosterol oxide found in ultra-processed foods, and its role in UC. Clinically, dietary analysis revealed a positive correlation between KS intake and Mayo score in UC patients (p < 0.05). Furthermore, in a C57BL/6 mouse model, KS was demonstrated to exacerbate dextran sulfate sodium (DSS)-induced colitis (p < 0.001). Mechanistically, KS impedes nuclear translocation of the N⁶-methyladenosine (m⁶A) demethylase AlkB homolog 5 (ALKBH5) (p < 0.05). This suppression downregulates expression of the glutamate-cysteine ligase modifier subunit (GCLM), impairing glutathione biosynthesis and ultimately triggering macrophage ferroptosis, as evidenced by increased levels of malondialdehyde (MDA), Fe2+, and reactive oxygen species (ROS) with statistical significance (p < 0.05). Crucially, ALKBH5 overexpression restored GCLM-mediated antioxidant defenses and mitigated ferroptosis in RAW264.7 cells (p < 0.05). Immunofluorescence confirmed KS-mediated suppression of the ALKBH5-GCLM axis in clinical specimens, exhibiting strong correlation with UC (p < 0.05). Our findings establish processed ultra-food-derived KS as a novel environmental driver of UC pathogenesis through regulation of macrophage ferroptosis. These results identify the KS-ALKBH5-GCLM-ferroptosis axis as a promising therapeutic target for UC intervention.
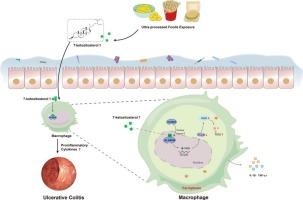
过量的超加工食品暴露通过巨噬细胞铁下垂加重溃疡性结肠炎
溃疡性结肠炎(UC)是一种全球流行的慢性炎症性疾病,越来越多地与现代饮食危害的环境暴露有关,特别是含有氧化植物甾醇的超加工食品。虽然已知工业食品加工技术和重复用油会增加植物甾醇氧化产物的形成,但它们对肠道炎症的生物学影响仍未被探索。这项研究的重点是7-酮谷甾醇(KS),在超加工食品中发现的最丰富的植物甾醇氧化物,及其在UC中的作用。临床上,饮食分析显示UC患者的KS摄入量与Mayo评分呈正相关(p <; 0.05)。此外,在C57BL/6小鼠模型中,KS被证明会加重葡聚糖硫酸钠(DSS)诱导的结肠炎(p <; 0.001)。在机制上,KS阻碍N⁶-甲基腺苷(m⁶A)去甲基化酶AlkB同源物5 (ALKBH5)的核易位(p <; 0.05)。这种抑制下调了谷氨酸-半胱氨酸连接酶修饰子亚基(GCLM)的表达,损害谷胱甘肽的生物合成,最终引发巨噬细胞铁凋亡,丙二醛(MDA)、铁离子(Fe2+)和活性氧(ROS)水平升高,差异有统计学意义(p <; 0.05)。至关重要的是,ALKBH5过表达恢复了gclm介导的抗氧化防御,减轻了RAW264.7细胞中的铁凋亡(p <; 0.05)。免疫荧光证实临床标本中ks介导的ALKBH5-GCLM轴抑制与UC有很强的相关性(p <; 0.05)。我们的研究结果表明,加工的超食物来源的KS通过调节巨噬细胞铁凋亡,成为UC发病的一种新的环境驱动因素。这些结果确定ks - alkbh5 - gclm -铁下垂轴是UC干预的一个有希望的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


