A Co-catalytic nanosystem based on molybdenum disulfide and Prussian blue for synergistic chemodynamic and photothermal therapy through mitochondrial damage and ferroptosis
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Chemodynamic therapy (CDT) is regarded as an emerging strategy with high specificity for tumor therapy by producing highly toxic reactive oxygen species (ROS) in tumor cells by a Fenton or Fenton-like reaction. Excessive ROS can cause mitochondrial damage and induce ferroptosis in cells, leading to the death of cancer cells. However, the generally low efficiency of the Fenton reaction has limited the effectiveness of CDT. Herein, two-dimensional MoS2 decorated with PB NPs is used as a co-catalyst to promote FeIII/FeII conversion and thus enhance the efficiency of the Fenton reaction. The photothermal properties of both MoS2 and PB NPs further enhance the Fenton reaction, eventually producing a large amount of ROS. Mitochondrial damage and ferroptosis caused by ROS are evidenced in vitro and in a tumor-bearing mouse model and jointly lead to a decrease in heat shock protein content, further enhancing the photothermal effect of PB NP/MoS2 nanosystem. This chemodynamic/photothermal synergistic therapy allows achieving good anticancer therapeutic effect.
Statement of significance
CDT is a promising cancer treatment that selectively generates toxic ROS to eliminate tumor cells. Nevertheless, its efficacy is often limited by the low efficiency of the Fenton reaction. This study presents a nanocomposite composed of MoS₂ nanosheets decorated with PB NPs, which enhances CDT by improving FeIII/FeII conversion and increasing ROS production. In addition, the photothermal properties of the material further amplify its therapeutic effects. In cell and animal models, this synergistic approach effectively induces mitochondrial damage and ferroptosis, thereby weakening the defenses of the cancer cells. This work provides a significant advancement in CDT, offering a more potent strategy for cancer therapy.
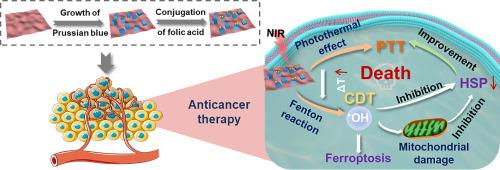
基于二硫化钼和普鲁士蓝的共催化纳米体系对线粒体损伤和铁凋亡的化学动力学和光热协同治疗。
化学动力疗法(CDT)是一种新兴的、具有高特异性的肿瘤治疗策略,它通过Fenton或Fenton样反应在肿瘤细胞中产生高毒性活性氧(ROS)。过量的ROS可引起线粒体损伤,诱导细胞铁下垂,导致癌细胞死亡。然而,Fenton反应的效率普遍较低,限制了CDT的有效性。本文以普鲁士蓝纳米粒子(PB NPs)修饰的二维二硫化钼作为助催化剂,促进FeIII/FeII的转化,从而提高Fenton反应的效率。MoS2和PB NPs的光热性质进一步增强了Fenton反应,最终产生大量ROS。体外和荷瘤小鼠模型证实了ROS引起的线粒体损伤和铁下沉,并共同导致热休克蛋白含量降低,进一步增强了PB NP/MoS2纳米系统的光热效应。这种化学动力/光热协同疗法可以达到良好的抗癌治疗效果。意义声明:化学动力疗法(CDT)是一种很有前途的癌症治疗方法,它选择性地产生有毒的活性氧(ROS)来消除肿瘤细胞。然而,其效果往往受到芬顿反应效率低的限制。本研究提出了一种由普鲁士蓝纳米粒子修饰的MoS 2纳米片组成的纳米复合材料,通过改善FeIII/FeII转化和增加ROS生成来增强CDT。此外,该材料的光热特性进一步增强了其治疗效果。在细胞和动物模型中,这种协同方法有效地诱导线粒体损伤和铁下垂,从而削弱癌细胞的防御。这项工作提供了CDT的重大进展,为癌症治疗提供了更有效的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


